Korean J Radiol.
2002 Dec;3(4):245-253. 10.3348/kjr.2002.3.4.245.
Saline-Enhanced Radiofrequency Thermal Ablation of the Lung: A Feasibility Study in Rabbits
- Affiliations
-
- 1Department of Radiology, Chonbuk National University Medical School, Korea. jmlshy@naver.com
- 2Department of Radiology, Seoul National University Hospital, Korea.
- 3Department of General Surgery, Chonbuk National University Medical School, Korea.
- 4Department of Surgical Pathology, Chonbuk National University Medical School, Korea.
- 5Department of Radiology, Yangi Hospital, Korea.
- KMID: 1118792
- DOI: http://doi.org/10.3348/kjr.2002.3.4.245
Abstract
OBJECTIVE
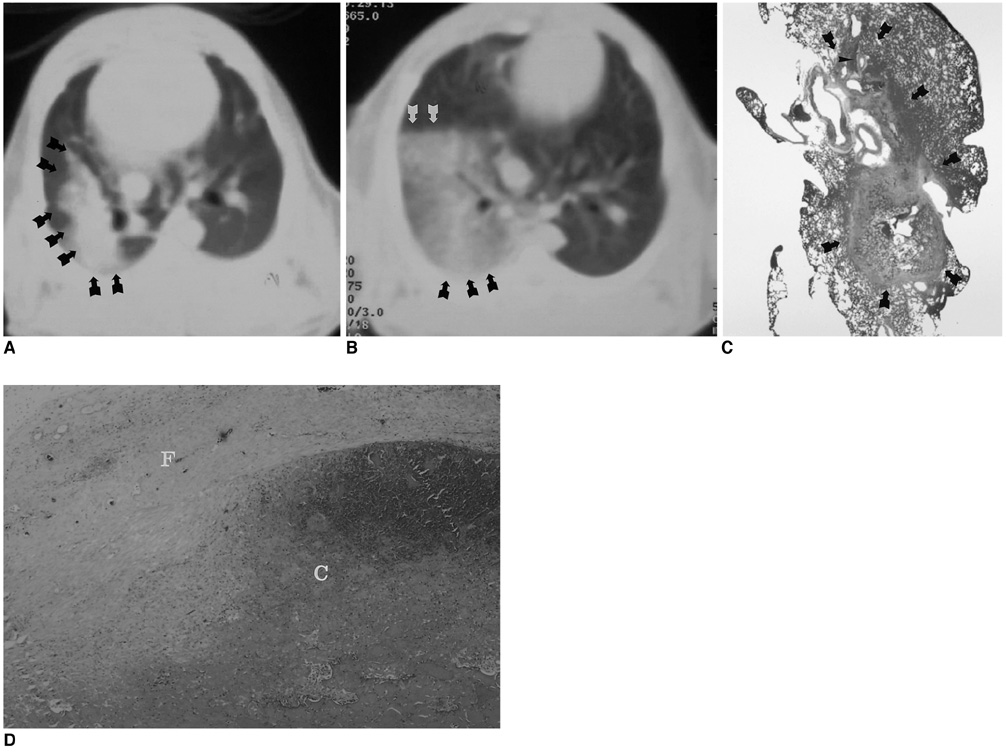
To assess the feasibility and safety of CT-guided percutaneous transthoracic radiofrequency ablation (RFA) with saline infusion of pulmonary tissue in rabbits. MATERIALS AND METHODS: Twenty-eight New Zealand White rabbits were divided into two groups: an RFA group (n=10) and a saline-enhanced RFA (SRFA) group (n=18). In the RFA group, percutaneous RFA of the lung was performed under CT guidance and using a 17-gauge internally cooled electrode. In the SRFA group, 1.5 ml of 0.9% saline was infused slowly through a 21-gauge, polyteflon-coated Chiba needle prior to and during RFA. Lesion size and the healing process were studied in rabbits sacrificed at times from the day following treatment to three weeks after, and any complications were noted. RESULTS: In the SRFA group, the mean diameter (12.5+/-1.6 mm) of acute RF lesions was greater than that of RFA lesions (8.5+/-1.4 mm) (p < .05). The complications arising in 12 cases were pneumothorax (n=8), thermal injury to the chest wall (n=2), hemothorax (n=1), and lung abscess (n=1). Although procedure-related complications tended to occur more frequently in the SRFA group (55.6%) than in the RFA group (20%), the difference was not statistically significant (p = .11). CONCLUSION: Saline-enhanced RFA of pulmonary tissue in rabbits produces more extensive coagulation necrosis than conventional RFA procedures, without adding substantial risk of serious complications.
MeSH Terms
Figure
Reference
-
1. Goldberg SN, Livraghi T, Solbiati L, Gazelle GS. Gazelle GS, Saini S, Mueller PR, editors. In-situ ablation of focal hepatic neoplasms. Hepatobiliary and pancreatic radiology: imaging and intervention. 1997. New York: Thieme;470–502.2. Curley SA, Izzo F, Ellis LM, Vauthey JN, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000. 232:381–391.3. Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999. 230:1–8.4. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.5. Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996. 167:759–768.6. Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis created by overlapping ablations. AJR Am J Roentgenol. 2002. 177:777–782.7. Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radiofrequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997. 202:195–203.8. Sironi S, Livraghi T, Meloni F, Cobelli FD, Ferrero C, Maschio AD. Small hepatocellular carcinoma treated with percutaneous RF ablation: MR imaging follow-up. AJR Am J Roentgenol. 1999. 173:1225–1229.9. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998. 80:815–821.10. Gervais DA, McGovern FJ, Wood BJ, et al. Radiofrequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000. 217:665–672.11. Jeffrey SS, Birdwell RL, Ikeda DM, et al. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg. 1999. 134:1064–1068.12. Anzai Y, Lufkin R, DeSalles A, et al. Preliminary experience with MR-guided thermal ablation of brain tumors. AJNR Am J Neuroradiol. 1995. 16:39–48.13. Zagoria RJ, Chen MY, Kavanagh PV, Torti FM. Radiofrequency ablation of lung metastases from renal cell carcinoma. J Urol. 2001. 166:1827–1828.14. Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000. 174:57–59.15. Goldberg SN, Gazelle GS, Compton CC, McLoud TC. Radiofrequency tissue ablation in the rabbit lung: efficacy and complications. Acad Radiol. 1995. 2:776–784.16. Goldberg SN, Gazelle GS, Compton CC, Mueller PR, McLoud TC. Radiofrequency tissue ablation of VX2 tumor nodules in the rabbit lung. Acad Radiol. 1996. 3:929–935.17. Miao Y, Ni Y, Bosmans H, et al. Radiofrequency ablation for eradication of pulmonary tumor in rabbits. J Surg Res. 2001. 99:265–271.18. Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radiofrequency tissue ablation in the treatment of liver metastases. Radiology. 1997. 202:205–210.19. Miao Y, Ni Y, Mulier S, et al. Ex-vivo experiment on radiofrequency liver ablation with saline infusion through a screw-tip cannulated electrode. J Surg Res. 1997. 71:19–24.20. Haaga JR. Haaga JR, Lanzieri CF. Interventional CT-guided procedures. Computed tomography and magnetic resonance imaging of the whole body. 1994. 3rd ed. St.Louis: Mosby;1572–1693.21. Goldberg SN, Stein M, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: optimization of pulsed-RF technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999. 10:907–916.22. Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001. 2:151–158.23. Ginsberg RJ, Vokes EE, Raben A. Pass HI, Mitchell JB, Johnson DH, Turrisi AT, editors. Cancer of the lung: non-small cell lung cancer. Cancer: principles and practice of oncology. 1996. 5th ed. Philadelphia: Lippincott-Raven;849–857.24. Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: a national cancer data base report. Cancer. 1999. 86:1867–1876.25. Downey RJ. Surgical management of lung cancer. J Thorac Imaging. 1999. 14:266–269.26. Moore DF Jr, Lee JS. Pass HD, Mitchell JB, Johnson DH, Turrisi AT, editors. Staging and prognostic factors: non-small cell lung cancer. Lung cancer: principles and practice. 1996. Philadelphia: Lippincott-Raven;481–494.27. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.28. Haines DE, Verow AF. Observations on electrode-tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation. 1990. 82:1034–1038.29. Geddes LA, Baker LE. The specific resistance of biological material-a compendium of data for the biomedical engineer and physiologist. Med Biol Eng. 1967. 5:271–293.30. Gazelle GS, Goldberg SN, Solbiati L, et al. Tumor ablation with radio-frequency energy. Radiology. 2000. 217:633–646.31. Miao Y, Ni Y, Yu J, Marchal G. A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol. 2000. 35:438–444.32. Boehm T, Malich A, Goldberg SN, et al. Radio-frequency tumor ablation: internally cooled electrode versus saline-enhanced technique in an aggressive rabbit tumor model. Radiology. 2002. 222:805–813.33. Goldberg SN, Ahmed M, Gazelle GS, et al. Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation phantom and porcine liver study. Radiology. 2001. 219:157–165.34. Shennib HA, Landreneau R, Mulder DS, et al. Video-assisted thoracoscopic wedge resection of T1 lung cancer in high-risk patients. Ann Surg. 1993. 218:555–560.35. Landreneau RJ, Sugarbaker D, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small cell lung cancer. J Thorac Cardiovasc Surg. 1997. 113:691–700.36. Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1995. 60:615–623.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Saline-enhanced Radiofrequency Electrocoagulation in Bovine Liver
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas
- Skin Burn after Laparoscopic Radiofrequency Thermal Ablation for Uterine Myoma : A case report
- Feasibility of Saline Infusion on the Liver Surface during Radiofrequency Ablation of Subcapsular Hepatic Tumor: An Experimental Study
- Percutaneous Radiofrequency Thermal Ablation of Lung VX2 Tumors in a Rabbit Model: Evaluation with Helical CT Findings for the Complete and Partal Ablation