Korean J Ophthalmol.
2009 Sep;23(3):188-192. 10.3341/kjo.2009.23.3.188.
Concentration of Vascular Endothelial Growth Factor After Intracameral Bevacizumab Injection in Eyes With Neovascular Glaucoma
- Affiliations
-
- 1Myung-Gok Eye Research Institute, Department of Ophthalmology, Konyang University, Kim's Eye Hospital, Seoul, Korea. t951101@ichannel.net
- KMID: 1115751
- DOI: http://doi.org/10.3341/kjo.2009.23.3.188
Abstract
- PURPOSE
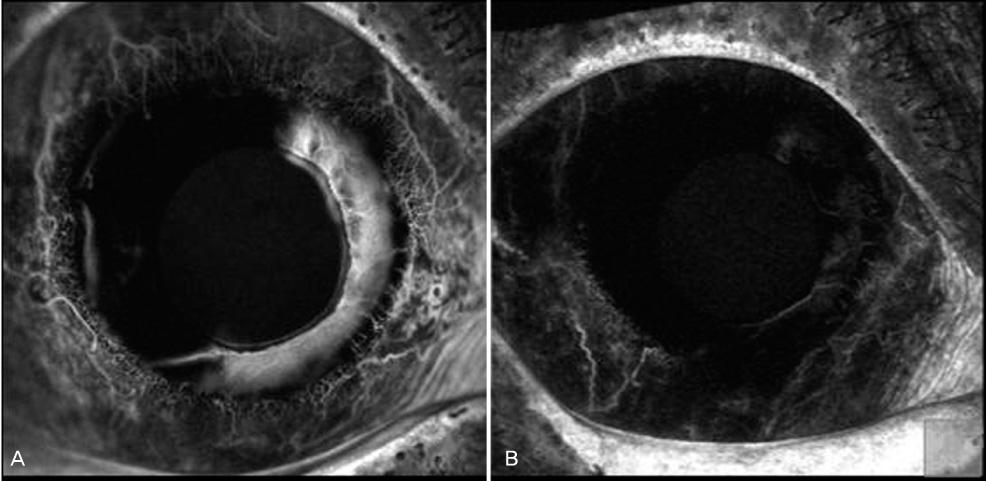
To study the concentration of vascular endothelial growth factor (VEGF) in the aqueous humor before and after intracameral injection of bevacizumab in eyes with neovascular glaucoma, and to detect the duration of an anti-VEGF effect of bevacizumab in the anterior chamber. METHODS: In this prospective interventional case series, 1.25 mg of bevacizumab was injected into the anterior chamber of five eyes in five neovascular glaucoma patients. Aqueous humor samples were obtained just before intracameral injection of bevacizumab and two weeks after injection. The concentrations of VEGF in the aqueous humor were measured using ELISA. To investigate corneal endothelial damage after intrecameral bevacizumab injection, specular microscopy was performed before injection and two weeks after injection. Slit lamp photo and iris fluorescent angiography was performed to determine the regression of iris neovascularization. RESULTS: After injection, substantial regression of neovascularization or fluorescein leakage was seen in all treated eyes. The VEGF concentrations in the aqueous humor in eyes with NVG were 1181.8+/-1248.3 pg/mL before intracameral injection of bevacizumab. Two weeks after injection, the VEGF concentrations decreased to 33.2+/-12.2 pg/mL (p=0.04, Wilcoxon signed rank test). There were no significant changes in IOP or corneal endothelial cells. CONCLUSIONS: Intracameral bevacizumab injection can remarkably reduce iris neovascularization in neovascular glaucoma patients. VEGF levels were significantly decreased two weeks after injection and corneal toxicity was not observed during short term follow-up.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal/*administration & dosage
Aqueous Humor/*metabolism
Eye
Glaucoma, Neovascular/*drug therapy/*metabolism
Humans
Injections
Middle Aged
Osmolar Concentration
Prospective Studies
Vascular Endothelial Growth Factor A/antagonists & inhibitors/*metabolism
Figure
Reference
-
1. Tripathi RC, Li J, Tripathi BJ, et al. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology. 1998. 105:232–237.2. Chalam KV, Guphta SK, Grover S, et al. Intracameral Avastin dramatically resolves iris neovascularization and reverses neovascular glaucoma. Eur J Ophthalmol. 2008. 18:255–262.3. Glaser BM. Extracellular modulating factors and the control of intraocular neovascularization. Arch Ophthalmol. 1988. 106:603–607.4. Sivalingam A, Kenney J, Brown GC, et al. Basic fibroblast growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1990. 108:869–872.5. Tripathi RC, Borisuth NSC, Li J, et al. Growth factors in the aqueous humor and their clinical significance. J Glaucoma. 1994. 3:248–258.6. Meyer-Schwickerath R, Pfeiffer A, Blum WF, et al. Vitreous levels of the insulin-like growth factors I and II, and the insulin-like growth factor binding proteins 2 and 3, increase in neovascular eye diseases: studies in nondiabetic and diabetic subjects. J Clin Invest. 1993. 92:2620–2625.7. Tripathi RC, Li J, Tripathi BJ, et al. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology. 1998. 105:232–237.8. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin formetastatic colorectal cancer. N Engl J Med. 2004. 350:2335–2342.9. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994. 331:1480–1487.10. Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular Glaucoma. J Glaucoma. 2007. 16:437–439.11. Sivack-Callcott JA, O'Day DM, Gass DM, et al. Evidence based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001. 108:1767–1776.12. Ng EW, Anthony AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005. 40:352–368.13. Tolentino MJ, Miller JW, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996. 114:964–970.14. Milko E, Domig Diego, Wolf-Schnurrbursch Ute, et al. Intravitreal Bevacizumab (Avastin®) in the Treatment of Neovascular Glaucoma. Am J Ophthalmol. 2006. 142:1054–1056.15. Maeng Hyosung, Kim Jinchul, Kee Changwon, et al. Intraviteal Bevacizumab (Avastin® Injection for the Treatment of Early-Stage Neovascular Glaucoma. J Korean Ophthalmol Soc. 2008. 49:696–700.16. Sawada O, Kawamura H, Kakinoki M, et al. Vascular Endothelial Growth Factor in Aqueous Humor Before and After Intravitreal Injection of Bevacizumab in Eyes With Diabetic Retinopathy. Arch Ophthalmol. 2007. 125:1363–1366.17. Chiang CC, Chen WL, Lin JM, Tsai YY. Effect of Bevacizumab on Human Corneal Endothelial Cells: A Six-month Follow-up Study. Am J Ophthalmol. 2008. 146:688–691.18. Yoeruek E, Spitzer MS, Tatar O, et al. Safety Profile of Bevacizumab on Cultured Human Corneal Cells. Cornea. 2007. 26:977–982.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Intracameral Bevacizumab for Treatment of Neovascular Glaucoma
- Long-Term Results of Ahmed Valve Implantation in Neovascular Glaucoma and the Effects of Intracameral Bevacizumab
- Comparision between Simultaneous Intracameral and Intravitreal Injection and Intravitreal Injection of Bevacizumab in Neovascular Glaucoma
- Effects of Intravitreal Injection of Bevacizumab or Ranibizumab on Systemic Circulation
- Subconjunctival Bevacizumab as an Adjunct to Trabeculectomy in Eyes with Refractory Glaucoma: A Case Series