Korean J Ophthalmol.
2011 Apr;25(2):90-97. 10.3341/kjo.2011.25.2.90.
Factors Influencing the Visual Acuity of Chronic Central Serous Chorioretinopathy
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. yykim@cu.ac.kr
- 2Casey Eye Institute, Oregon Health and Science University, Portland, OR, USA.
- KMID: 1111869
- DOI: http://doi.org/10.3341/kjo.2011.25.2.90
Abstract
- PURPOSE
To investigate correlated factors on final visual acuity in conjunction with fluorescein angiography (FA) and optical coherence tomography (OCT) findings of chronic central serous chorioretinopathy (CSCR).
METHODS
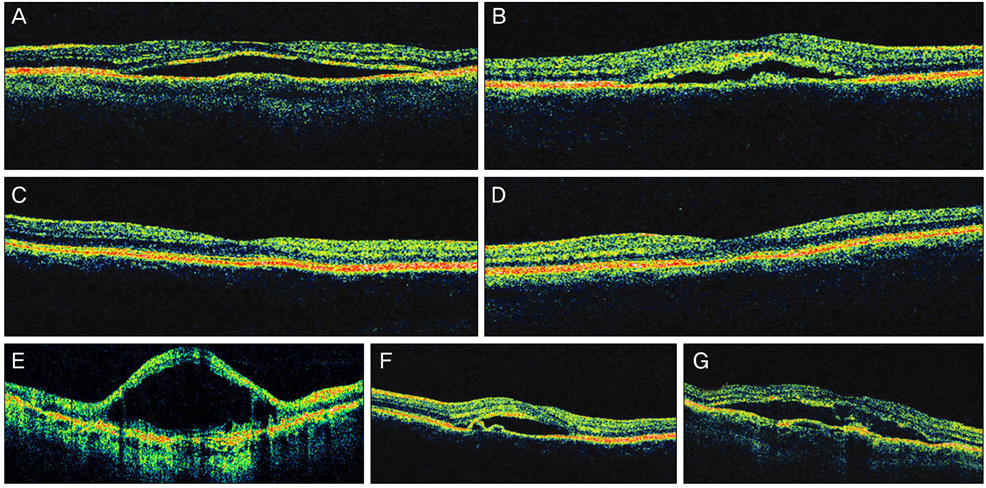
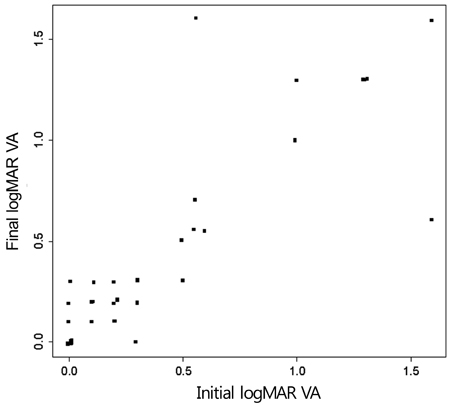
Twenty-four patients (36 eyes) with typical findings of chronic CSCR based on medical records, FA and OCT results were enrolled in this study. We investigated demographic findings, initial and final visual acuity (VA), and some typical findings of FA including the type of leakage pattern, the existence of a gravitational tract and an abnormal hyperfluorescent area centered on the fovea. We also investigated OCT findings to examine serous retinal detachment, outer photoreceptor layer (OPRL) preservation, continuity of the inner segment (IS) and the outer segment (OS) of the photoreceptor layer in case of macular attachment, and other typical findings. The converted logarithm of the minimum angle of resolution VA was used to investigate the statistical correlation with these FA and OCT findings.
RESULTS
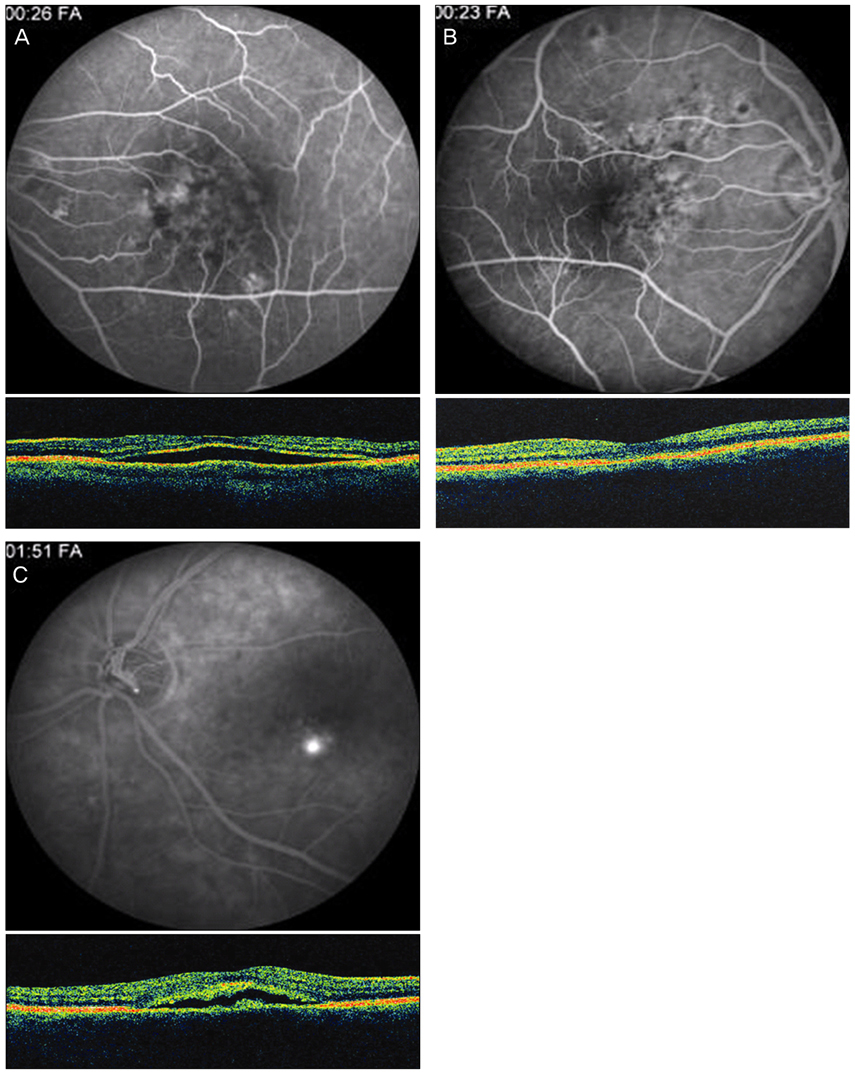
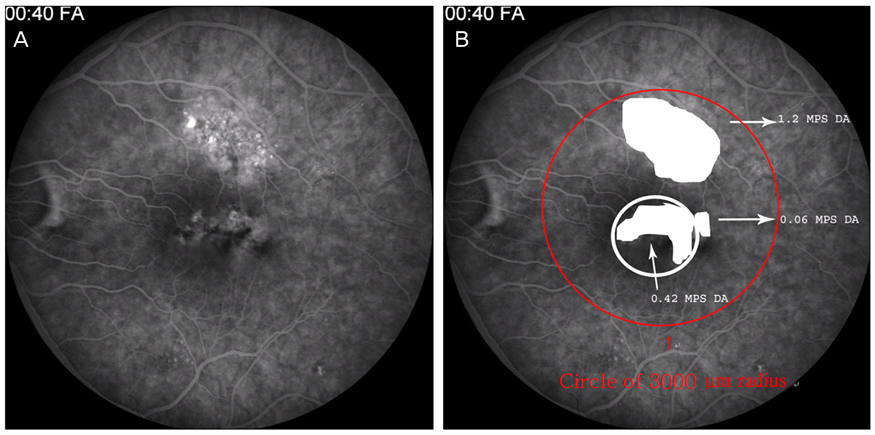
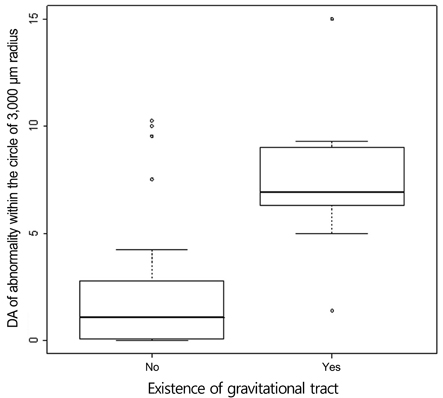
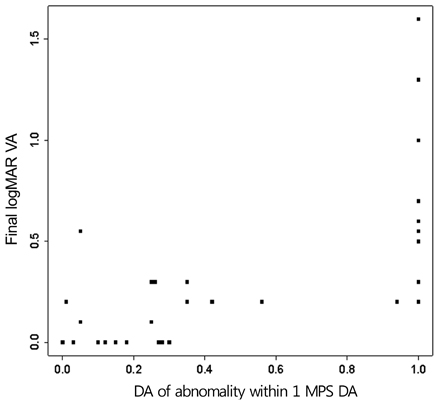
An abnormal hyperfluorescent area within 1 macular photocoagulation study disc area on FA and cystoid degeneration on OCT were correlated with poor final VA of less than 20 / 40. However, the preserved OPRL and the continuity of IS / OS junction were correlated with a good final VA of 0.5 or more.
CONCLUSIONS
These specific findings could be associated with recurrent or persistent subretinal fluid and could be important parameters of decision for treatment.
Keyword
MeSH Terms
-
Adult
Aged
Central Serous Chorioretinopathy/complications/diagnosis/*physiopathology
Chronic Disease
Female
Fluorescein Angiography
Follow-Up Studies
Fundus Oculi
Humans
Male
Middle Aged
Retina/*pathology
Retinal Detachment/diagnosis/etiology/physiopathology
Retrospective Studies
Severity of Illness Index
Tomography, Optical Coherence
*Visual Acuity
Figure
Cited by 1 articles
-
Outer Retinal Layers Alterations in Chronic Central Serous Chorioretinopathy: Spectral Domain-OCT and Fundus Autofluorescence Findings
In Seok Oh, Ji Hye Jang
J Korean Ophthalmol Soc. 2016;57(5):763-771. doi: 10.3341/jkos.2016.57.5.763.
Reference
-
1. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967. 63:Suppl. 1–139.2. Schatz H, Madeira D, Johnson RN, McDonald HR. Central serous chorioretinopathy occurring in patients 60 years of age and older. Ophthalmology. 1992. 99:63–67.3. Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992. 81:379–386.4. Klein ML, Van Buskirk EM, Friedman E, et al. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974. 91:247–250.5. Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984. 68:815–820.6. Lu JG, Friberg TR. Idiopathic central serous retinopathy in China: a report of 600 cases (624 eyes) treated by acupuncture. Ophthalmic Surg. 1987. 18:608–611.7. Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001. 79:417–421.8. Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002. 47:431–448.9. Lafaut BA, Salati C, Priem H, De Laey JJ. Indocyanine green angiography is of value for the diagnosis of chronic central serous chorioretinopathy in elderly patients. Graefes Arch Clin Exp Ophthalmol. 1998. 236:513–521.10. Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984. 91:1554–1572.11. Iida T, Hagimura N, Sato T, Kishi S. Evaluation of central serous chorioretinopathy with optical coherence tomography. Am J Ophthalmol. 2000. 129:16–20.12. Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003. 23:1–7.13. Gomolin JE. Choroidal neovascularization and central serous chorioretinopathy. Can J Ophthalmol. 1989. 24:20–23.14. Spitznas M, Huke J. Number, shape, and topography of leakage points in acute type I central serous retinopathy. Graefes Arch Clin Exp Ophthalmol. 1987. 225:437–440.15. Yamada K, Hayasaka S, Setogawa T. Fluorescein-angiographic patterns in patients with central serous chorioretinopathy at the initial visit. Ophthalmologica. 1992. 205:69–76.16. Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol. 2005. 89:562–564.17. Hirami Y, Tsujikawa A, Sasahara M, et al. Alterations of retinal pigment epithelium in central serous chorioretinopathy. Clin Experiment Ophthalmol. 2007. 35:225–230.18. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005. 139:87–99.19. Prunte C. Indocyanine green angiographic findings in central serous chorioretinopathy. Int Ophthalmol. 1995. 19:77–82.20. Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996. 121:26–34.21. Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986. 9:37–41.22. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002. 22:19–24.23. Yap EY, Robertson DM. The long-term outcome of central serous chorioretinopathy. Arch Ophthalmol. 1996. 114:689–692.24. Levine R, Brucker AJ, Robinson F, et al. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989. 96:854–859.25. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996. 103:2070–2079.26. Gackle HC, Lang GE, Freissler KA, Lang GK. Central serous chorioretinopathy: clinical, fluorescein angiography and demographic aspects. Ophthalmologe. 1998. 95:529–533.27. Wang M, Sander B, la Cour M, Larsen M. Clinical characteristics of subretinal deposits in central serous chorioretinopathy. Acta Ophthalmol Scand. 2005. 83:691–696.28. Wong R, Chopdar A, Brown M. Five to 15 year follow-up of resolved idiopathic central serous chorioretinopathy. Eye (Lond). 2004. 18:262–268.29. Bujarborua D, Chatterjee S, Choudhury A, et al. Fluorescein angiographic features of asymptomatic eyes in central serous chorioretinopathy. Retina. 2005. 25:422–429.30. Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002. 133:787–793.31. Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008. 86:126–145.32. Erickson PA, Fisher SK, Anderson DH, et al. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983. 24:927–942.33. Bill A, Tornquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K. 1980. 100:332–336.34. Anderson DH, Guerin CJ, Erickson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986. 27:168–183.35. Sakai T, Calderone JB, Lewis GP, et al. Cone photoreceptor recovery after experimental detachment and reattachment: an immunocytochemical, morphological, and electrophysiological study. Invest Ophthalmol Vis Sci. 2003. 44:416–425.36. Taban M, Boyer DS, Thomas EL, Taban M. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol. 2004. 137:1073–1080.37. Chung SE, Kang JH, Kang SW. Chronic central serous chorioretinopathy: photodynamic therapy. J Korean Ophthalmol Soc. 2007. 48:279–284.38. Lai TY, Chan WM, Li H, et al. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006. 90:869–874.39. Reinke MH, Canakis C, Husain D, et al. Verteporfin photodynamic therapy retreatment of normal retina and choroid in the cynomolgus monkey. Ophthalmology. 1999. 106:1915–1923.40. Peyman GA, Kazi AA, Unal M, et al. Problems with and pitfalls of photodynamic therapy. Ophthalmology. 2000. 107:29–35.41. Zacks DN, Ezra E, Terada Y, et al. Verteporfin photodynamic therapy in the rat model of choroidal neovascularization: angiographic and histologic characterization. Invest Ophthalmol Vis Sci. 2002. 43:2384–2391.42. Paskowitz DM, Nune G, Yasumura D, et al. BDNF reduces the retinal toxicity of verteporfin photodynamic therapy. Invest Ophthalmol Vis Sci. 2004. 45:4190–4196.43. Cayouette M, Behn D, Sendtner M, et al. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998. 18:9282–9293.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Chronic Central Serous Chorioretinopathy Associated with Pituitary Adenoma
- A Case of Idiopathic Multiple Central Serous Chorioretinopathy
- The Use fulness of OCT[Optical Coherence Tomography]for the Diagnosis of Central Serous Choriore tinopathy
- Photodynamic Therapy With Verteporfin using Half Fluence for Chronic Central Serous Chorioretinopathy
- Clinical Aspect of Central Serous Chorioretinopathy