Yonsei Med J.
2011 Sep;52(5):851-855. 10.3349/ymj.2011.52.5.851.
IL-10 is Predominantly Produced by CD19(low)CD5(+) Regulatory B Cell Subpopulation: Characterisation of CD19 (high) and CD19(low) Subpopulations of CD5(+) B cells
- Affiliations
-
- 1Department of Paediatrics, College of Medicine, Chungnam National University, Daejeon, Korea.
- 2Department of Animal Biotechnology, College of Animal Bioscience & Technology, Konkuk University, Seoul, Korea.
- 3Division of Allergy and Clinical Immunology, Department of Paediatrics, Chungnam National University Hospital, Daejeon, Korea. admyth@naver.com
- 4Department of Immunology, College of Medicine, Konkuk University, Chungju, Korea.
- 5Department of Food and Nutrition, College of Human Ecology, Hanyang University, Seoul, Korea.
- KMID: 1108080
- DOI: http://doi.org/10.3349/ymj.2011.52.5.851
Abstract
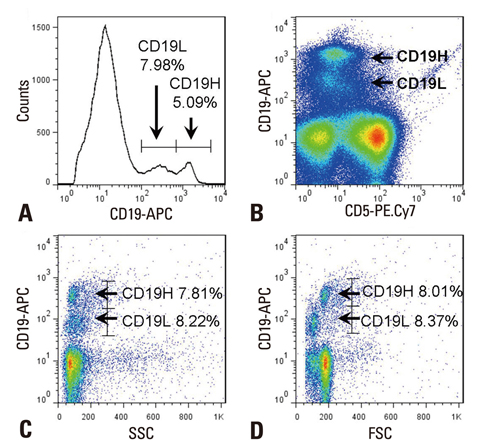
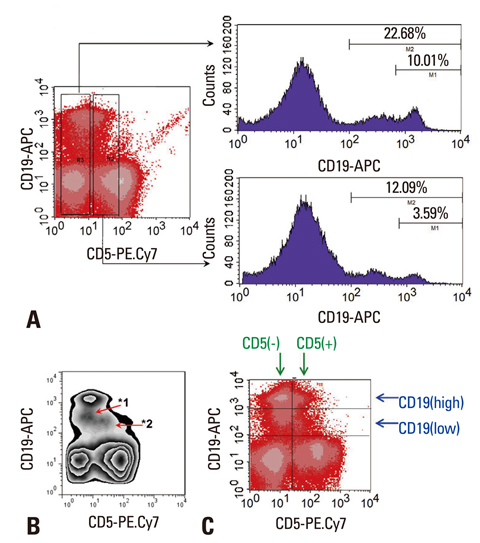
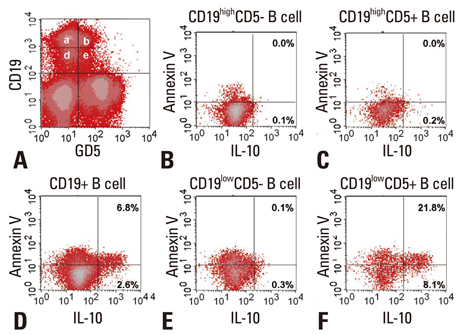
- IL-10 production by CD19(+)CD5(+) B cells was investigated, by determining the expression levels of CD19, a classical B cell marker. Peripheral mononuclear cells were stained with fluorescence-conjugated anti-CD5, anti-CD19, anti-IL-10, and Annexin V. Interestingly, IL-10-producing B cells were found to be localised within the CD19(low)CD5(+) B cell subset. Apoptotic changes were also observed mainly in CD19(low) cells among B cells. Thus, CD5(+) B cells should be classified as CD19(high) and CD19(low) cells, and the immunological significance of CD19 for the IL-10 production by CD5(+) B cells requires further studies.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Regulatory B Cells Are Inversely Associated with Disease Activity in Rheumatoid Arthritis
Jinhyun Kim, Hyun Ji Lee, In Seol Yoo, Seong Wook Kang, Jae Ho Lee
Yonsei Med J. 2014;55(5):1354-1358. doi: 10.3349/ymj.2014.55.5.1354.
Reference
-
1. Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006. 176:705–710.
Article2. Gieni RS, Umetsu DT, DeKruyff RH. Ly1- (CD5-) B cells produce interleukin (IL)-10. Cell Immunol. 1997. 175:164–170.3. Lee JH, Noh J, Noh G, Kim HS, Mun SH, Choi WS, et al. Allergen-specific B cell subset responses in cow's milk allergy of late eczematous reactions in atopic dermatitis. Cell Immunol. 2010. 262:44–51.
Article4. Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009. 182:7459–7472.
Article5. Fujimoto M, Sato S. B cell signaling and autoimmune diseases: CD19/CD22 loop as a B cell signaling device to regulate the balance of autoimmunity. J Dermatol Sci. 2007. 46:1–9.
Article6. Tedder TF, Isaacs CM. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes. A new member of the immunoglobulin superfamily. J Immunol. 1989. 143:712–717.7. Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, et al. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006. 168:812–821.
Article8. Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010. 40:2686–2691.
Article9. Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells. Trends Immunol. 2008. 29:34–40.
Article10. Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS, et al. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in Cow Milk Allergy. Cell Immunol. 2010. 264:143–149.
Article11. Taylor DK, Ito E, Thorn M, Sundar K, Tedder T, Spatz LA. Loss of tolerance of anti-dsDNA B cells in mice overexpressing CD19. Mol Immunol. 2006. 43:1776–1790.
Article12. Sato S, Steeber DA, Jansen PJ, Tedder TF. CD19 expression levels regulate B lymphocyte development: human CD19 restores normal function in mice lacking endogenous CD19. J Immunol. 1997. 158:4662–4669.13. Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, et al. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol. 2005. 175:8011–8023.
Article14. Sato S, Miller AS, Howard MC, Tedder TF. Regulation of B lymphocyte development and activation by the CD19/CD21/CD81/Leu 13 complex requires the cytoplasmic domain of CD19. J Immunol. 1997. 159:3278–3287.15. Shoham T, Rajapaksa R, Boucheix C, Rubinstein E, Poe JC, Tedder TF, et al. The tetraspanin CD81 regulates the expression of CD19 during B cell development in a postendoplasmic reticulum compartment. J Immunol. 2003. 171:4062–4072.
Article16. Fujimoto M, Poe JC, Jansen PJ, Sato S, Tedder TF. CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation. J Immunol. 1999. 162:7088–7094.17. DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010. 1183:38–57.
Article18. Garaud S, Le Dantec C, de Mendoza AR, Mageed RA, Youinou P, Renaudineau Y. IL-10 production by B cells expressing CD5 with the alternative exon 1B. Ann N Y Acad Sci. 2009. 1173:280–285.
Article19. Díaz-Alderete A, Crispin JC, Vargas-Rojas MI, Alcocer-Varela J. IL-10 production in B cells is confined to CD154+ cells in patients with systemic lupus erythematosus. J Autoimmun. 2004. 23:379–383.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Presence of Foxp3-expressing CD19(+)CD5(+) B Cells in Human Peripheral Blood Mononuclear Cells: Human CD19(+)CD5(+)Foxp3(+) Regulatory B Cell (Breg)
- Change of Peripheral Blood CD5+ B Lymphocytes in Early Neonatal Period
- Increased CD5 + B Cells in Neonatal Infections
- CD20dim T Cell Expression in Rheumatoid Arthritis
- Regulatory B Cells and Allergic Diseases