J Vet Sci.
2011 Jun;12(2):121-126. 10.4142/jvs.2011.12.2.121.
Evaluation of the estrogenic effects of dietary perinatal Trifolium pratense
- Affiliations
-
- 1Department of Histology and Embryology, Faculty of Veterinary Medicine, Istanbul University, 34320 Avcilar, Istanbul, Turkey. emryat@gmail.com
- KMID: 1106553
- DOI: http://doi.org/10.4142/jvs.2011.12.2.121
Abstract
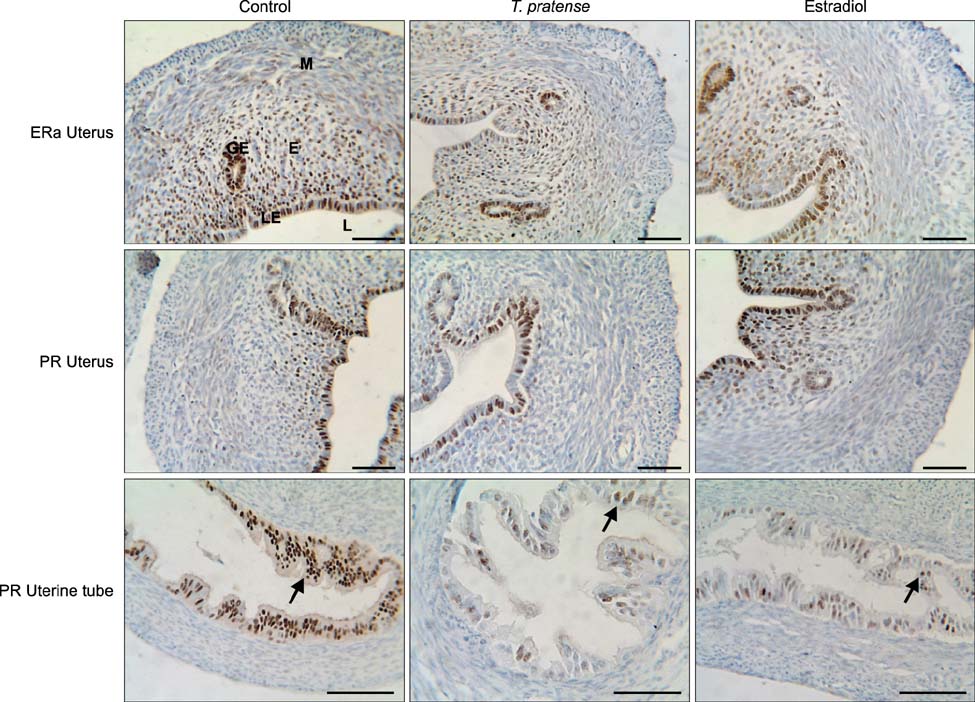
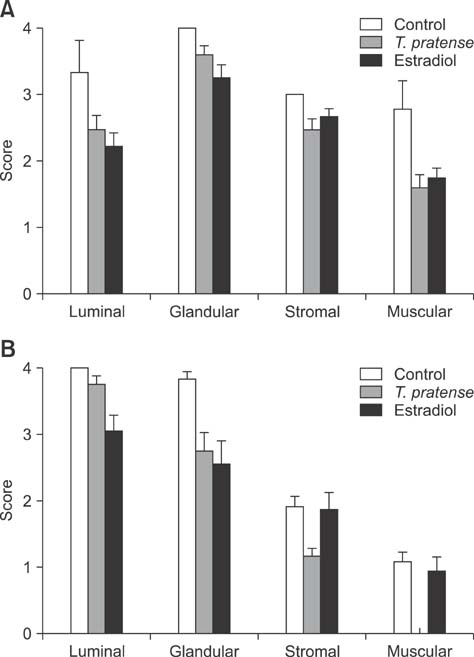
- This study was designed to investigate the potential estrogenic effects of perinatal dietary phytoestrogens on the rat uterus. Pregnant rats were divided to three groups provided the following diets: (1) rat chow, (2) rat chow with 7.5% Trifolium (T.) pratense, or (3) rat chow supplemented with 17beta-estradiol (0.5 mg/kg). The dams in each group were kept on the same diet during pregnancy and lactation. Female offspring were euthanized on day 21 at which time body and organ weights were recorded and tissue samples were taken for histology. Immunohistochemistry was performed to detect estrogen receptor alpha (ERalpha) and progesterone receptor (PR) levels. Our results revealed estrogen-like biological effects of perinatal T. pratense exposure. Relative uterus and ovary weights in the experimental groups were increased compared to control. The number of uterine glands and luminal epithelium heights were also increased. However, there were no statistically significant changes detected in the immunostaining intensity of ERalpha and PR between the groups.
MeSH Terms
-
Animals
Animals, Suckling
Body Weight/drug effects
Estrogen Receptor alpha/*metabolism
Female
Immunohistochemistry
Isoflavones/*pharmacology
Lactation
Maternal Exposure
Organ Size/drug effects
Phytoestrogens/*pharmacology
Plant Components, Aerial/chemistry
Pregnancy
Rats
Rats, Wistar
Receptors, Progesterone/*metabolism
Trifolium/*chemistry
Uterus/*drug effects
Figure
Reference
-
1. Adams NR. Detection of the effects of phytoestrogens on Sheep and Cattle. J Anim Sci. 1995. 73:1509–1515.
Article2. Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999. 140:5364–5373.
Article3. Beck V, Rohr U, Jungbauer A. Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? J Steroid Biochem Mol Biol. 2005. 94:499–518.
Article4. Booth NL, Overk CR, Yao P, Totura S, Deng Y, Hedayat AS, Bolton JL, Pauli GF, Farnsworth NR. Seasonal variation of red clover (Trifolium pratense L., Fabaceae) isoflavones and estrogenic activity. J Agric Food Chem. 2006. 54:1277–1282.
Article5. Booth NL, Piersen CE, Banuvar S, Geller SE, Shulman LP, Farnsworth NR. Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: a literature review. Menopause. 2006. 13:251–264.
Article6. Boué SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agric Food Chem. 2003. 51:2193–2199.
Article7. Burdette JE, Liu J, Lantvit D, Lim E, Booth N, Bhat KP, Hedayat S, Van Breemen RB, Constantinou AI, Pezzuto JM, Farnsworth NR, Bolton JL. Trifolium pratense (red clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J Nutr. 2002. 132:27–30.
Article8. Coon JT, Pittler MH, Ernst E. Trifolium pratense isoflavones in the treatment of menopausal hot flushes: a systematic review and meta-analysis. Phytomedicine. 2007. 14:153–159.
Article9. Cotroneo MS, Wang J, Eltoum IA, Lamartiniere CA. Sex steroid receptor regulation by genistein in the prepubertal rat uterus. Mol Cell Endocrinol. 2001. 173:135–145.
Article10. Foth D, Cline JM. Effects of mammalian and plant estrogens on mammary glands and uteri of macaques. Am J Clin Nutr. 1998. 68:6 Suppl. 1413S–1417S.
Article11. Jarred RA, Keikha M, Dowling C, McPherson SJ, Clare AM, Husband AJ, Pedersen JS, Frydenberg M, Risbridger GP. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol Biomarkers Prev. 2002. 11:1689–1696.12. Kallela K, Heinonen K, Saloniemi H. Plant estrogens; The cause of decreased fertility in cows. A case report. Nord Vet Med. 1984. 36:124–129.13. Katzenellenbogen BS, Nardulli AM, Read LD. Estrogen regulation of proliferation and hormonal modulation of estrogen and progesterone receptor biosynthesis and degradation in target cells. Prog Clin Biol Res. 1990. 322:201–211.14. Krenn L, Unterrieder I, Ruprechter R. Quantification of isoflavones in red clover by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002. 777:123–128.
Article15. Krenn L, Paper DH. Inhibition of angiogenesis and inflammation by an extract of red clover (Trifolium pratense L.). Phytomedicine. 2009. 16:1083–1088.
Article16. Lethaby AE, Brown J, Marjoribanks J, Kronenberg F, Roberts H, Eden J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007. CD001395.
Article17. Milgrom E, Thi L, Atger M, Baulieu EE. Mechanisms regulating the concentration and the conformation of progesterone receptor(s) in the uterus. J Biol Chem. 1973. 248:6366–6374.
Article18. Overk CR, Guo J, Chadwick LR, Lantvit DD, Minassi A, Appendino G, Chen SN, Lankin DC, Farnsworth NR, Pauli GF, van Breemen RB, Bolton JL. In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. Chem Biol Interact. 2008. 176:30–39.
Article19. Parvu E. Trifolium pratense for breast disease: a case series. Homeopathy. 2004. 93:45–50.
Article20. Sabudak T, Guler N. Trifolium L. - A review on its phytochemical and pharmacological profile. Phytother Res. 2009. 23:439–446.21. Saloniemi H, Wähälä K, Nykänen-Kurki P, Kallela K, Saastamoinen I. Phytoestrogen content and estrogenic effect of legume fodder. Proc Soc Exp Biol Med. 1995. 208:13–17.
Article22. Saviranta NMM, Anttonen MJ, von Wright A, Karjalainen RO. Red clover (Trifolium pratense L.) isoflavones: determination of concentrations by plant stage, flower colour, plant part and cultivar. J Sci Food Agric. 2008. 88:125–132.
Article23. Sivesind E, Seguin P. Effects of the environment, cultivar, maturity, and preservation method on red clover isoflavone concentration. J Agric Food Chem. 2005. 53:6397–6402.
Article24. Wang W, Tanaka Y, Han Z, Higuchi CM. Proliferative response of mammary glandular tissue to formononetin. Nutr Cancer. 1995. 23:131–140.
Article25. Wuttke W, Jarry H, Seidlová-Wuttke D. Isoflavones-Safe food additives or dangerous drugs. Ageing Res Rev. 2007. 6:150–188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trifolium pratense induces apoptosis through caspase pathway in FaDu human hypopharynx squamous carcinoma cells
- Additive Estrogenic Activities of the Binary Mixtures of Four Estrogenic Chemicals in Recombinant Yeast Expressing Human Estrogen Receptor
- The Effects of Gender, Obesity Rate, Nutrition Knowledge and Dietary Attitude on the Dietary Self-Efficacy of Adolescents
- Effects of Mycorrhizal and Endophytic Fungi on Plant Community: a Microcosm Study
- Requirement of Metabolic Activation for Estrogenic Activity of Pueraria mirifica