J Vet Sci.
2008 Jun;9(2):169-175. 10.4142/jvs.2008.9.2.169.
In vivo morphological and antigenic characteristics of Photobacterium damselae subsp. piscicida
- Affiliations
-
- 1Laboratory of Aquatic Animal Diseases, College of Veterinary Medicine, Gyeongsang National University, Jinju 660-701, Korea. jungts@gsnu.ac.kr
- 2Aquatic Vaccine Unit, Institute of Aquaculture, University of Stirling, Stirling, FK9 4LA, Scotland, UK.
- 3Dipartimento di Scienze della Produzione Animale, Faculta di Medicina Veterinaria, Universita degli studi di Udine, 20B 33100 Udine, Italy.
- KMID: 1106232
- DOI: http://doi.org/10.4142/jvs.2008.9.2.169
Abstract
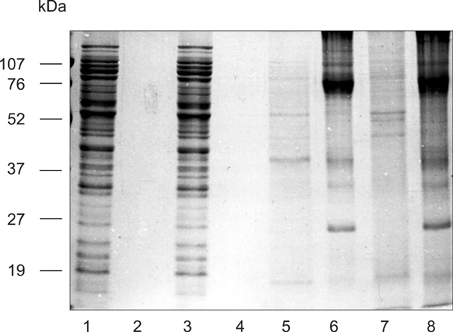
- The present study was conducted to examine the morphology and antigenicity of Photobacterium damselae subsp. piscicida by culturing the bacterium in vivo in the peritoneal cavity of sea bass (Dicentrarchus labrax) within dialysis bags with either a low molecular weight (LMW) cut-off of 25 kDa or a high molecular weight (HMW) cut-off of 300 kDa. Differences were observed in the growth rate between the bacteria cultured in vivo or in vitro. Bacteria cultured in vivo were smaller and produced a capsular layer, which was more prominent in bacteria cultured in the HMW bag. Antigenicity was examined by Western blot analysis using sera from sea bass injected with live Ph. d. subsp. piscicida. The sera recognised bands at 45 and 20 kDa in bacteria cultured in vivo in the LMW bag. Bacteria cultured in vivo in the HMW bag did not express the 45 kDa band when whole cell extracts were examined, although the antigen was present in their extracellular products. In addition, these bacteria had a band at 18 kDa rather than 20 kDa. Differences in glycoprotein were also evident between bacteria cultured in vitro and in vivo. Bacteria cultured in vitro in LMW and HMW bags displayed a single 26 kDa band. Bacteria cultured in the LMW bag in vivo displayed bands at 26 and 27 kDa, while bacteria cultured in vivo in the HMW bag possessed only the 27 kDa band. These bands may represent sialic acid. The significance of the changes observed in the bacterium's structure and antigenicity when cultured in vivo is discussed.
MeSH Terms
-
Animals
Antigenic Variation/*genetics
Antigens, Bacterial/genetics/*immunology
Bass/*immunology/microbiology
Blotting, Western
Carbohydrates/analysis
Electrophoresis, Polyacrylamide Gel
Membranes, Artificial
Microscopy, Electron, Transmission
N-Acetylneuraminic Acid/genetics/*immunology
Photobacterium/genetics/*immunology/ultrastructure
Figure
Reference
-
1. Arijo S, Balebona C, Martinez-Manzanares E, Moriñigo MA. Immune response of gilt-head seabream (Sparus aurata) to antigens from Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 2004. 16:65–70.
Article2. Arijo S, Borrego JJ, Zorrilla I, Balebona MC, Moriñigo MA. Role of the capsule of Photobacterium damsela subsp. piscicida in protection against phagocytosis and killing by gilt-head seabream (Sparus aurata, L) macrophages. Fish Shellfish Immunol. 1998. 8:63–72.
Article3. Bakopoulos V, Adams A, Richards RH. The effect of iron limitation growth conditions on the cell and extracellular components of the fish pathogen Pasteurella piscicida. J Fish Dis. 1997. 20:297–305.
Article4. Bakopoulos V, Hanif A, Poulos K, Galeotti M, Adams A, Dimitriadis GJ. The effect of in vivo growth on the cellular and extracellular components of the marine bacterial pathogen Photobacterium damsela subsp. piscicida. J Fish Dis. 2004. 27:1–14.5. Bakopoulos V, Pearson M, Volpatti D, Gousmani L, Adams A, Galeotti M, Dimitriadis GJ. Investigation of media formulations promoting differential antigen expression by Photobacterium damsela ssp. piscicida and recognition by sea bass, Dicentrarchus labrax (L.), immune sera. J Fish Dis. 2003. 26:1–13.
Article6. Bakopoulos V, Volpatti D, Gusmani L, Galeotti M, Adams A, Dimitriadis GJ. Vaccination trials of sea bass, Dicentrarchus labrax (L.), against Photobacterium damsela subsp. piscicida, using novel vaccine mixtures. J Fish Dis. 2003. 26:77–90.
Article7. Bonet R, Magariños B, Romalde JL, Simon-Pujol MD, Toranzo AE, Congregado F. Capsular polysaccharide expressed by Pasteurella piscicida grown in vitro. FEMS Microbiol Lett. 1994. 124:285–289.
Article8. Brown MR, Anwar H, Costerton JW. Surface antigens in vivo: a mirror for vaccine development. Can J Microbiol. 1988. 34:494–498.9. do Vale A, Afonso A, Silva MT. The professional phagocytes of sea bass (Dicentrarchus labrax L.): cytochemical characterisation of neutrophils and macrophages in the normal and inflamed peritoneal cavity. Fish Shellfish Immunol. 2002. 13:183–198.
Article10. do Vale A, Ellis AE, Silva MT. Electron microscopic evidence that expression of capsular polysaccharide by Photobacterium damselae subsp. piscicida is dependent on iron availability and growth phase. Dis Aquat Organ. 2001. 44:237–240.
Article11. do Vale A, Magariños B, Romalde JL, Lemos ML, Ellis AE, Toranzo AE. Binding of haemin by the fish pathogen Photobacterium damselae subsp. piscicida. Dis Aquat Organ. 2002. 48:109–115.
Article12. Díaz-Rosales P, Chabrillón M, Moriñigo MA, Balebona MC. Survival against exogenous hydrogen peroxide of Photobacterium damselae subsp. piscicida under different culture conditions. J Fish Dis. 2003. 26:305–308.13. Garduño RA, Kay WW. Capsulated cells of Aeromonas salmonicida grown in vitro have different functional properties than capsulated cells grown in vivo. Can J Microbiol. 1995. 41:941–945.
Article14. Garduño RA, Thornton JC, Kay WW. Aeromonas salmonicida grown in vivo. Infect Immunol. 1993. 61:3854–3862.15. Garrote A, Bonet R, Merino S, Simon-Pujol MD, Congregado F. Occurrence of a capsule in Aeromonas salmonicida. FEMS Microbiol Lett. 1992. 95:127–132.16. Hawke JP, Thune RL, Cooper RK, Judice E, Kelly-Smith M. Molecular and phenotypic characterization of strains of Photobacterium damselae subsp. piscicida isolated from hybrid striped bass cultured in Louisiana, USA. J Aquat Anim Health. 2003. 15:189–201.
Article17. Juiz-Río S, Osorio CR, Lemos ML. Identification and characterisation of the fur genes in Photobacterium damselae ssp. piscicida and ssp damselae. Dis Aquat Organ. 2004. 58:151–156.18. Jung TS, Thompson KD, Adams A. A comparison of sialic acid between different isolates of Photobacterium damselae subsp. piscicida. Fish Pathol. 2001. 36:217–224.
Article19. Jung TS, Thompson KD, Volpatti D, Galeotti M, Adams A. Variation in the molecular weight of Photobacterium damselae subsp. piscicida antigens when cultured under different conditions in vitro. J Vet Sci. 2007. 8:255–261.
Article20. Kawakami H, Sakai M. Comparison of susceptibility of seven fishes to Photobacterium damsela subsp. piscicida. Bull Eur Assoc Fish Pathol. 1999. 19:153–155.21. Kawakami H, Shinohara N, Fukuda Y, Yamashita H, Kihara H, Sakai M. The efficacy of lipopolysaccharide mixed chloroform-killed cell (LPS-CKC) bacterin of Pasteurella piscicida on Yellowtail, Seriola quinqueradiata. Aquaculture. 1997. 154:95–105.
Article22. Kawakami H, Shinohara N, Sakai M. The non-specific immunostimulation and adjuvant effects of Vibrio anguillarum bacterin, M-glucan, chitin and Freund's complete adjuvant against Pasteurella piscicida infection in yellowtail. Fish Pathol. 1998. 33:287–292.
Article23. Magariños B, Romalde JL, Lemos ML, Barja JL, Toranzo AE. Iron uptake by Pasteurella piscicida and its role in pathogenicity for fish. Appl Environ Microbiol. 1994. 60:2990–2998.
Article24. Magariños B, Romalde JL, Lopez-Romalde S, Morinigo MA, Toranzo AE. Pathobiological characterization of Photobacterium damselae subsp. piscicida strains isolated from cultured sole (Solea senegalensis). Bull Eur Assoc Fish Pathol. 2003. 23:183–190.25. Magariños B, Romalde JL, Santos Y, Casal JF, Barja JL, Toranzo AE. Vaccination trials on gilt-head sea bream (Sparus aurata) against Pasteurella piscicida. Aquaculture. 1994. 120:201–208.
Article26. Magariños B, Santos Y, Romalde JL, Rivas C, Barja JL, Toranzo AE. Pathogenic activities of live cells and extracellular products of the fish pathogen Pasteurella piscicida. J Gen Microbiol. 1992. 138:2491–2498.27. Moriñigo MA, Romalde JL, Chabrillon M, Magariños B, Arijo S, Balebona MC, Toranzo AE. Effectiveness of a divalent vaccine for gilthead sea bream (Sparus aurata) against Vibrio alginolyticus and Photobacterium damselae subsp. piscicida. Bull Eur Assoc Fish Pathol. 2002. 22:298–303.28. Nitzan S, Shwartsburd B, Heller ED. The effect of growth medium salinity of Photobacterium damselae subsp. piscicida on the immune response of hybrid bass (Morone saxatilis × M. chrysops). Fish Shellfish Immunol. 2004. 16:107–116.
Article29. Nitzan S, Shwartsburd B, Vaiman R, Heller ED. Some characteristics of Photobacterium damselae ssp. piscicida isolated in Israel during outbreaks of pasteurellosis in hybrid bass (Morone saxatilis × M. chrysops). Bull Eur Assoc Fish Pathol. 2001. 21:77–80.30. Romalde JL. Photobacterium damselae subsp. piscicida: an integrated view of a bacterial fish pathogen. Int Microbiol. 2002. 5:3–9.
Article31. Salyers AA, Whitt DD. Bacterial Pathogenesis: A Molecular Approach. 1994. 1st ed. Washington DC: ASM Press;63–72.32. Thornton JC, Garduño RA, Carlos SJ, Kay WW. Novel antigens expressed by Aeromonas salmonicida grown in vivo. Infect Immun. 1993. 61:4582–4589.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Variation in the molecular weight of Photobacterium damselae subsp. piscicida antigens when cultured under different conditions in vitro
- Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria
- In vivo pefloxacin-resistant Campylobacter fetus responsible for gastro-intestinal infection and bacteremia associated with arthritis of the hip
- Analysis of antigenic characteristics of Rickettsia tsutsugamushi Boryong strain and antigenic heterogeneity of Rickettsia tsutsugamushi using monoclonal antibodies
- Influence of temperature on the antigenic changes of virus-like particles