J Vet Sci.
2008 Jun;9(2):133-144. 10.4142/jvs.2008.9.2.133.
The C-terminal variable domain of LigB from Leptospira mediates binding to fibronectin
- Affiliations
-
- 1Department of Population Medicine and Diagnostic Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853, USA. yc42@cornell.edu
- KMID: 1106228
- DOI: http://doi.org/10.4142/jvs.2008.9.2.133
Abstract
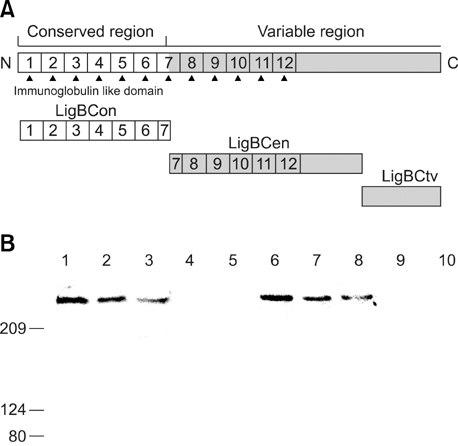
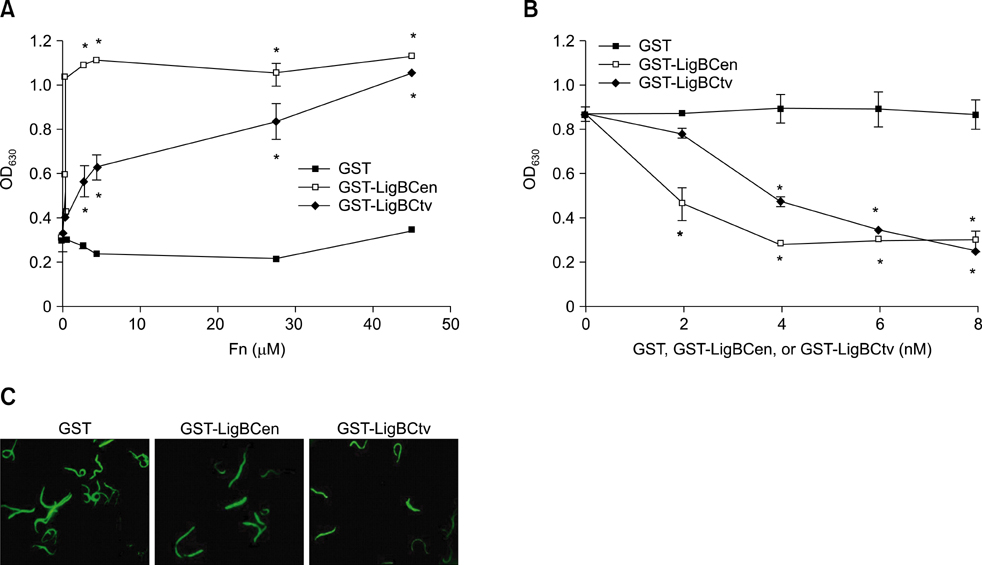
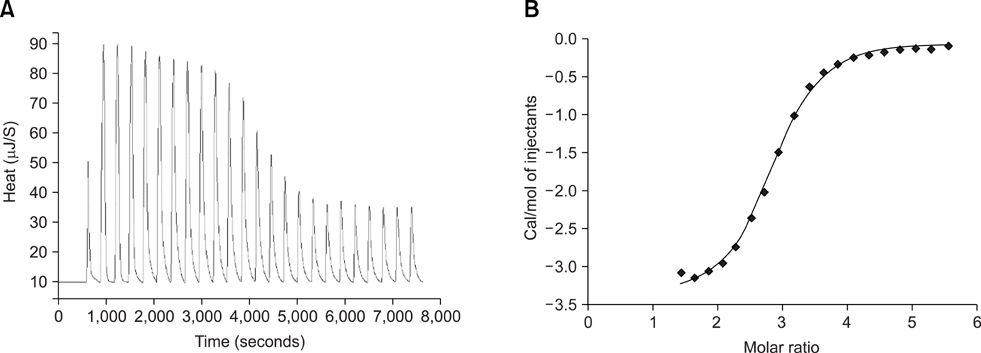
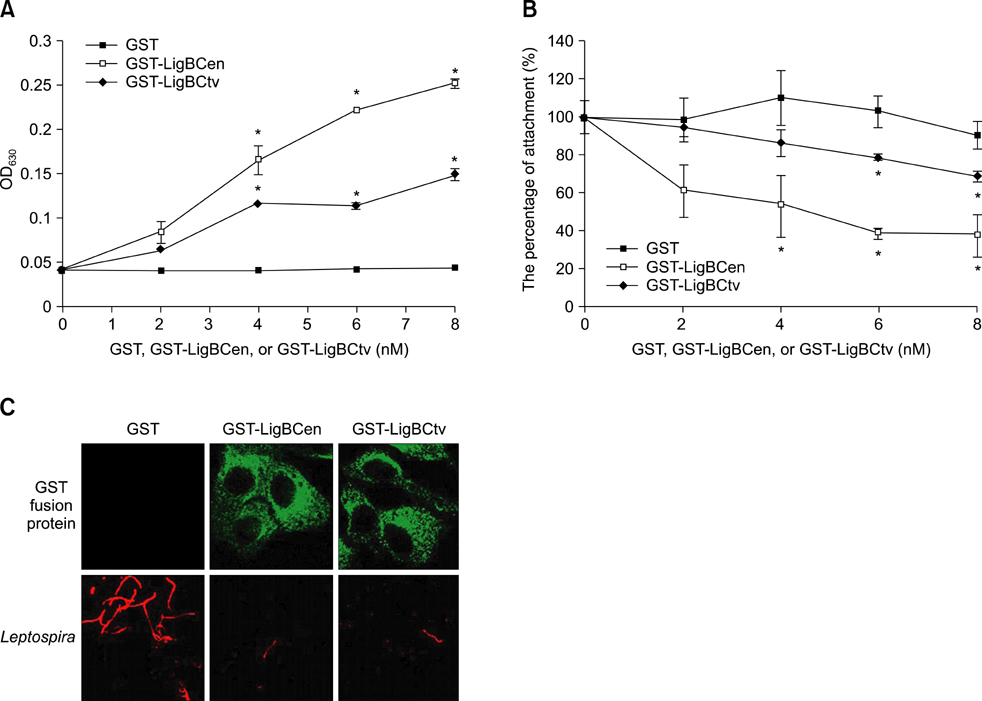
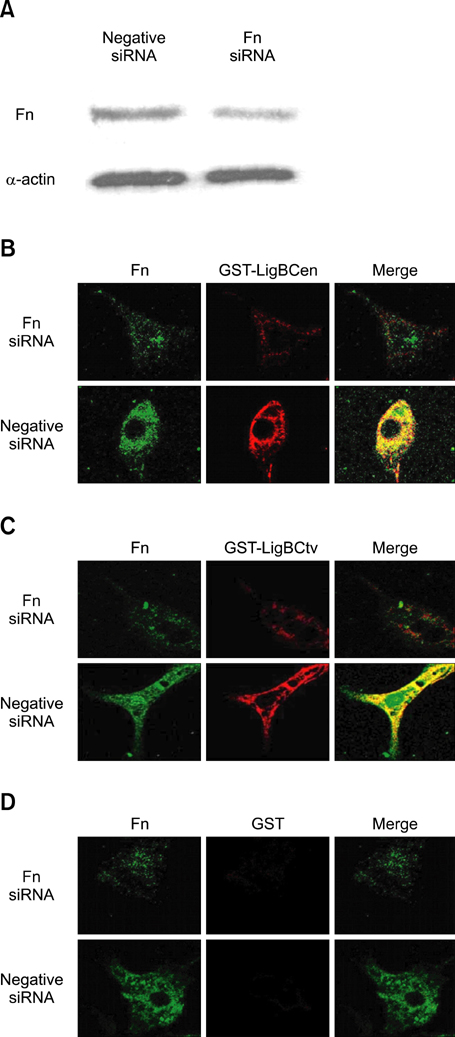
- Adhesion through microbial surface components that recognize adhesive matrix molecules is an essential step in infection for most pathogenic bacteria. In this study, we report that LigB interacts with fibronectin (Fn) through its variable region. A possible role for LigB in bacterial attachment to host cells during the course of infection is supported by the following observations: (i) binding of the variable region of LigB to Madin-Darby canine kidney (MDCK) cells in a dose-dependent manner reduces the adhesion of Leptospira, (ii) inhibition of leptospiral attachment to Fn by the variable region of LigB, and (iii) decrease in binding of the variable region of LigB to the MDCK cells in the presence of Fn. Furthermore, we found a significant reduction in binding of the variable region of LigB to Fn using small interfering RNA (siRNA). Finally, the isothermal titration calorimetric results confirmed the interaction between the variable region of LigB and Fn. This is the first report to demonstrate that LigB binds to MDCK cells. In addition, the reduction of Fn expression in the MDCK cells, by siRNA, reduced the binding of LigB. Taken together, the data from the present study showed that LigB is a Fn-binding protein of pathogenic Leptospira spp. and may play a pivotal role in Leptospira-host interaction during the initial stage of infection.
MeSH Terms
-
Animals
Antigens, Bacterial/*genetics/metabolism
Cell Line
Dogs
Enzyme-Linked Immunosorbent Assay
Fibronectins/*metabolism
Immunoglobulin Variable Region/genetics/*metabolism
Leptospira/*genetics/metabolism
Microscopy, Confocal
Protein Binding/*genetics
*Protein Structure, Tertiary
RNA, Small Interfering/genetics
Figure
Reference
-
1. Barbosa AS, Abreu PA, Neves FO, Atzingen MV, Watanabe MM, Vieira ML, Morais ZM, Vasconcellos SA, Nascimento AL. A newly identified leptospiral adhesin mediates attachment to laminin. Infect Immun. 2006. 74:6356–6364.
Article2. Barocchi MA, Ko AI, Reis MG, McDonald KL, Riley LW. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but nonintracellular pathogen. Infect Immun. 2002. 70:6926–6932.
Article3. Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, Cullen PA, Davis J, Johnson M, Kuczek E, Alt DP, Peterson-Burch B, Coppel RL, Rood JI, Davies JK, Adler B. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci USA. 2006. 103:14560–14565.
Article4. Cameron CE, Brouwer NL, Tisch LM, Kuroiwa JM. Defining the interaction of the Treponema pallidum adhesin Tp0751 with laminin. Infect Immun. 2005. 73:7485–7494.
Article5. Cameron CE, Brown EL, Kuroiwa JM, Schnapp LM, Brouwer NL. Treponema pallidum fibronectin-binding proteins. J Bacteriol. 2004. 186:7019–7022.6. Choy HA, Kelley MM, Chen TL, Moller AK, Matsunaga J, Haake DA. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect Immun. 2007. 75:2441–2450.
Article7. Coburn J, Fischer JR, Leong JM. Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol Microbiol. 2005. 57:1182–1195.
Article8. Darnell J, Lodish H, Baltimore D. Molecular Cell Biology. 1990. 2nd ed. New York: Scientific American Books;802–824.9. Edwards AM, Jenkinson HF, Woodward MJ, Dymock D. Binding properties and adhesion-mediating regions of the major sheath protein of Treponema denticola ATCC 35405. Infect Immun. 2005. 73:2891–2898.
Article10. Faine SB, Adher B, Bolin C, Perolat P. Leptospira and Leptospirosis. 1999. 2nd ed. Medbourne: MedSci;67–71.11. Fischer JR, LeBlanc KT, Leong JM. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect Immun. 2006. 74:435–441.
Article12. Grab DJ, Givens C, Kennedy R. Fibronectin-binding activity in Borrelia burgdorferi. Biochim Biophys ACTA. 1998. 1407:135–145.13. Ingham KC, Brew S, Vaz D, Sauder DN, McGavin MJ. Interaction of Staphylococcus aureus fibronectin-binding protein with fibronectin: affinity, stoichiometry, and modular requirements. J Biol Chem. 2004. 279:42945–42953.
Article14. Isberg RR, Voorhis DL, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987. 50:769–778.
Article15. Ito T, Yanagawa R. leptospiral attachment to extracellular matrix of mouse fibroblast (L929) cells. Vet Microbiol. 1987. 15:89–96.
Article16. Ito T, Yanagawa R. Leptospiral attachment to four structural components of extracellular matrix. Nippon juigaku zasshi. 1987. 49:875–882.
Article17. Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990. 87:7839–7843.
Article18. Jiang ST, Chiang HC, Cheng MH, Yang TP, Chuang WJ, Tang MJ. Role of fibronectin deposition in cystogenesis of Madin-Darby canine kidney cells. Kidney Intl. 1999. 56:92–103.
Article19. Kim JH, Singvall J, Schwarz-Linek U, Johnson BJ, Potts JR, Hook M. BBK32, a fibronectin binding MSCRAMM from Borrelia burgdorferi, contains a disordered region that undergoes a conformational change on ligand binding. J Biol Chem. 2004. 279:41706–41714.
Article20. Konkel ME, Christensen JE, Keech AM, Monteville MR, Klena JD, Garvis SG. Identification of a fibronectin-binding domain within the Campylobacter jejuni CadF protein. Mol Microbiol. 2005. 57:1022–1035.
Article21. Kopp PA, Schmitt M, Wellensiek HJ, Blobel H. Isolation and characterization of fibronectin-binding sites of Borrelia garinii N34. Infect Immun. 1995. 63:3804–3808.
Article22. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001. 14:296–326.
Article23. Li X, Liu X, Beck DS, Kantor FS, Fikrig E. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun. 2006. 74:3305–3313.
Article24. Lin YP, Chang YF. A domain of the Leptospira LigB contributes to high affinity binding of fibronectin. Biochem Biophys Res Commun. 2007. 362:443–448.
Article25. Matsunaga J, Barocchi MA, Croda J, Young TA, Sanchez Y, Siqueira I, Bolin CA, Reis MG, Riley LW, Haake DA, Ko AI. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol Microbiol. 2003. 49:929–945.26. Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, Haake DA. Response of Leptospira interrogans to Physiologic Osmolarity: Relevance in Signaling the Environment-to-Host Transition. Infect Immun. 2007. 75:2864–2874.27. Matsunaga J, Sanchez Y, Xu X, Haake DA. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect Immun. 2005. 73:70–78.
Article28. May M, Papazisi L, Gorton TS, Geary SJ. Identification of fibronectin-binding proteins in Mycoplasma gallisepticum strain R. Infect Immun. 2006. 74:1777–1785.
Article29. Meites E, Jay MT, Deresinski S, Shieh WJ, Zaki SR, Tompkins L, Smith DS. Reemerging leptospirosis, California. Emerg Infect Dis. 2004. 10:406–412.30. Merien F, Truccolo J, Baranton G, Perolat P. Identification of a 36-kDa fibronectin-binding protein expressed by a virulent variant of Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol Lett. 2000. 185:17–22.31. Nascimento AL, Ko AI, Martins EA, Monteiro-Vitorello CB, Ho PL, Haake DA, Verjovski-Almeida S, Hartskeerl RA, Marques MV, Oliveira MC, Menck CF, Leite LC, Carrer H, Coutinho LL, Degrave WM, Dellagostin OA, El-Dorry H, Ferro ES, Ferro MI, Furlan LR, Gamberini M, Giglioti EA, Goes-Neto A, Goldman GH, Goldman MH, Harakava R, Jeronimo SM, Junqueira-de-Azevedo IL, Kimura ET, Kuramae EE, Lemos EG, Lemos MV, Marino CL, Nunes LR, de Oliveira RC, Pereira GG, Reis MS, Schriefer A, Siqueira WJ, Sommer P, Tsai SM, Simpson AJ, Ferro JA, Camargo LE, Kitajima JP, Setubal JC, Van Sluys MA. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol. 2004. 186:2164–2172.
Article32. Nyberg P, Sakai T, Cho KH, Caparon MG, Fassler R, Bjorck L. Interactions with fibronectin attenuate the virulence of Streptococcus pyogenes. EMBO J. 2004. 23:2166–2174.33. Palaniappan RU, Chang YF, Hassan F, McDonough SP, Pough M, Barr SC, Simpson KW, Mohammed HO, Shin S, McDonough P, Zuerner RL, Qu J, Roe B. Expression of leptospiral immunoglobulin-like protein by Leptospira interrogans and evaluation of its diagnostic potential in a kinetic ELISA. J Med Microbiol. 2004. 53:975–984.
Article34. Palaniappan RU, Chang YF, Jusuf SS, Artiushin S, Timoney JF, McDonough SP, Barr SC, Divers TJ, Simpson KW, McDonough PL, Mohammed HO. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect Immun. 2002. 70:5924–5930.
Article35. Palaniappan RU, McDonough SP, Divers TJ, Chen CS, Pan MJ, Matsumoto M, Chang YF. Immunoprotection of recombinant leptospiral immunoglobulin-like protein A against Leptospira interrogans serovar Pomona infection. Infect Immun. 2006. 74:1745–1750.
Article36. Parma AE, Cerone SI, Sansinanea SA. Biochemical analysis by SDS-PAGE and western blotting of the antigenic relationship between Leptospira and equine ocular tissues. Vet Immunol Immunopathol. 1992. 33:179–185.
Article37. Pikas DS, Brown EL, Gurusiddappa S, Lee LY, Xu Y, Hook M. Decorin-binding sites in the adhesin DbpA from Borrelia burgdorferi: a synthetic peptide approach. J Biol Chem. 2003. 278:30920–30926.38. Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998. 30:1003–1015.
Article39. Probert WS, Kim JH, Hook M, Johnson BJ. Mapping the ligand-binding region of Borrelia burgdorferi fibronectin-binding protein BBK32. Infect Immun. 2001. 69:4129–4133.
Article40. Raibaud S, Schwarz-Linek U, Kim JH, Jenkins HT, Baines ER, Gurusiddappa S, Hook M, Potts JR. Borrelia burgdorferi binds fibronectin through a tandem beta-zipper, a common mechanism of fibronectin binding in staphylococci, streptococci, and spirochetes. J Biol Chem. 2005. 280:18803–18809.
Article41. Rathinam SR, Rathnam S, Selvaraj S, Dean D, Nozik RA, Namperumalsamy P. Uveitis associated with an epidemic outbreak of leptospirosis. Am J Ophthalmol. 1997. 124:71–79.42. Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, Xu H, Zhang YX, Xiong H, Lu G, Lu LF, Jiang HQ, Jia J, Tu YF, Jiang JX, Gu WY, Zhang YQ, Cai Z, Sheng HH, Yin HF, Zhang Y, Zhu GF, Wan M, Huang HL, Qian Z, Wang SY, Ma W, Yao ZJ, Shen Y, Qiang BQ, Xia QC, Guo XK, Danchin A, Saint Girons I, Somerville RL, Wen YM, Shi MH, Chen Z, Xu JG, Zhao GP. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003. 422:888–893.43. Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nature biotechnol. 2004. 22:326–330.
Article44. Rzomp KA, Scholtes LD, Briggs BJ, Whittaker GR, Scidmore MA. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Iimmun. 2003. 71:5855–5870.
Article45. Schorey JS, Holsti MA, Ratliff TL, Allen PM, Brown EJ. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol Microbiol. 1996. 21:321–329.
Article46. Schroder A, Schroder B, Roppenser B, Linder S, Sinha B, Fassler R, Aepfelbacher M. Staphylococcus aureus fibronectin binding protein-A induces motile attachment sites and complex actin remodeling in living endothelial cells. Mol Biol Cell. 2006. 17:5198–5210.47. Schwarz-Linek U, Pilka ES, Pickford AR, Kim JH, Hook M, Campbell ID, Potts JR. High affinity streptococcal binding to human fibronectin requires specific recognition of sequential F1 modules. J Biol Chem. 2004. 279:39017–39025.48. Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, Briggs JA, Gough TS, Hook M, Campbell ID, Potts JR. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature. 2003. 423:177–181.
Article49. Segura ER, Ganoza CA, Campos K, Ricaldi JN, Torres S, Silva H, Cespedes MJ, Matthias MA, Swancutt MA, Lopez Linan R, Gotuzzo E, Guerra H, Gilman RH, Vinetz JM. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin Infect Dis. 2005. 40:343–351.
Article50. Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Hook M, Skare JT. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006. 59:1591–1601.
Article51. Shi J, Scita G, Casanova JE. WAVE2 signaling mediates invasion of polarized epithelial cells by Salmonella typhimurium. J Biol Chem. 2005. 280:29849–29855.
Article52. Thomas DD, Higbie LM. In vitro association of leptospires with host cells. Infect Immun. 1990. 58:581–585.
Article53. Vendrame F, Segni M, Grassetti D, Tellone V, Augello G, Trischitta V, Torlontano M, Dotta F. Impaired caspase-3 expression by peripheral T cells in chronic autoimmune thyroiditis and in autoimmune polyendocrine syndrome-2. J Clin Endocrinol Metab. 2006. 91:5064–5068.
Article54. Verma A, Hellwage J, Artiushin S, Zipfel PF, Kraiczy P, Timoney JF, Stevenson B. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect Immun. 2006. 74:2659–2666.
Article55. Wann ER, Gurusiddappa S, Hook M. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem. 2000. 275:13863–13871.
Article56. Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, Underhill DM, Kirschning CJ, Wagner H, Aderem A, Tobias PS, Ulevitch RJ. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nature immunol. 2001. 2:346–352.
Article57. Xu Q, Yan B, Li S, Duan C. Fibronectin binds insulin-like growth factor-binding protein 5 and abolishes Its ligand-dependent action on cell migration. J Biol Chem. 2004. 279:4269–4277.
Article58. Yang CW, Wu MS, Pan MJ. Leptospirosis renal disease. Nephrol Dialysis Transplant. 2001. 16:Suppl 5. 73–77.
Article59. Yang CW, Wu MS, Pan MJ, Hsieh WJ, Vandewalle A, Huang CC. The Leptospira outer membrane protein LipL32 induces tubulointerstitial nephritis-mediated gene expression in mouse proximal tubule cells. J Am Soc Nephrol. 2002. 13:2037–2045.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The biologic effect of fibrin-binding synthetic oligopeptide on periodontal ligament cells
- Molecular Cloning of Novel Clathrin Assembly Protein Gene from Rat Brain
- Study on antigenic analysis and serial antibody titration by using leptospira interrogans isolated at Chungchongbukdo
- Identification of new serovar yeonchon and hongchon belonging to leptospira interrogans icterohaemorrhagiae serogroup
- Role of Androgen Receptor in Prostate Cancer: A Review