J Vet Sci.
2007 Dec;8(4):361-368. 10.4142/jvs.2007.8.4.361.
Changes of biomarkers with oral exposure to benzo(a)pyrene, phenanthrene and pyrene in rats
- Affiliations
-
- 1National Veterinary Research and Quarantine Service, Anyang 430-824, Korea. jeongsh@nvrqs.go.kr
- 2College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- KMID: 1106216
- DOI: http://doi.org/10.4142/jvs.2007.8.4.361
Abstract
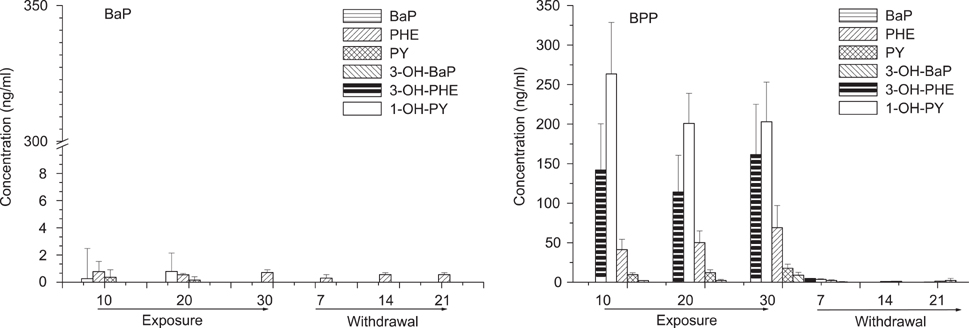
- Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants present in air and food. Among PAHs, benzo(a)pyrene(BaP), phenanthrene (PH) and pyrene (PY) are considered to be important for their toxicity or abundance. To investigate the changes of biomarkers after PAH exposure, rats were treated with BaP (150 microgram/kg) alone or with PH (4,300 microgram/kg) and PY (2,700 microgram/kg) (BPP group) by oral gavage once per day for 30 days. 7-ethoxyresorufin-O-deethylase activity in liver microsomal fraction was increased in only BaP groups. The highest concentration (34.5 ng/g) of BaP, was found in muscle of rats treated with BaP alone at 20 days of treatment; it was 23.6 ng/g in BPP treated rats at 30 days of treatment. The highest PH concentration was 47.1 ng/g in muscle and 118.8 ng/g in fat, and for PY it was 29.7 ng/g in muscle and 219.9 ng/g in fat, in BPP groups. In urine, 114-161 ng/ml 3-OH-PH was found, while PH was 41-69 ng/ml during treatment. 201-263 ng/ml 1-OH-PY was found, while PH was 9-17 ng/ml in urine. The level of PY, PH and their metabolites in urine was rapidly decreased after withdrawal of treatment. This study suggest that 1-OH-PY in urine is a sensitive biomarker for PAHs; it was the most highly detected marker among the three PAHs and their metabolites evaluated during the exposure period and for 14 days after withdrawal.
Keyword
MeSH Terms
-
Adipose Tissue/chemistry/drug effects
Animals
Benzo(a)pyrene/analysis/metabolism/*toxicity
Biological Markers/metabolism/urine
Blood Chemical Analysis
Body Weight/drug effects
Cytochrome P-450 CYP1A1/metabolism
Environmental Pollutants/blood/metabolism/*toxicity/urine
Female
Liver/drug effects/enzymology
Lymphocytes/drug effects/metabolism
Muscle, Skeletal/drug effects/metabolism
Organ Size/drug effects
Phenanthrenes/blood/metabolism/*toxicity/urine
Pyrenes/analysis/metabolism/*toxicity
Rats
Rats, Sprague-Dawley
Time Factors
Figure
Reference
-
1. Alexandrov K, Rojas M, Geneste O, Castegnaro M, Camus AM, Petruzzelli S, Giuntini C, Bartsch H. An improved fluorometric assay for dosimetry of benzo(a)pyrene diol-epoxide-DNA adducts in smokers' lung: comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992. 52:6248–6253.2. Arfsten DP, Schaeffer DJ, Muneny DC. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: a review. Ecotoxicol Environ Saf. 1996. 33:1–24.
Article3. Arif JM, Shappell N, Sikka HC, Kumar S, Gupta RC. 32P-Postlabeling analysis of lipophilic DNA adducts resulting from interaction with (±)-3-hydroxy-trans-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro-benzo[a]pyrene. Chem Biol Interact. 1999. 118:87–97.
Article4. Bosveld ATC, de Bie PAF, van den Brink NW, Jongepier H, Klomp AV. In vitro EROD induction equivalency factor for the 10 PAHs generally monitored in risk assessment studies in the Netherlands. Chemosphere. 2002. 49:75–83.
Article5. Buchet JP, Gennart JP, Mercado-Calderon F, Delavignette JP, Cupers L, Lauwerys R. Evaluation of exposure to polycyclic aromatic hydrocarbons in a coke production and a graphite electrode manufacturing plant: assessment of urinary excretion of 1-hydroxypyrene as a biological indicator of exposure. Br J Ind Med. 1992. 49:761–768.
Article6. Buckley TJ, Loiy PJ. An examination of the time course from human dietary exposure to polycyclic aromatic hydrocarbons to urinary elimination of 1-hydroxypyrene. Br J Ind Med. 1992. 49:113–124.
Article7. Bulter JP, Post GB, Lioy PJ, Waldman JM, Greenberg A. Assessment of carcinogenic risk from personal exposure to benzo(a)pyrene in the total human environmental exposure study (THEES). Air Waste. 1993. 43:970–977.
Article8. Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985. 34:3337–3345.
Article9. Chen BH, Wang CY, Chiu CP. Evaluation of analysis of polycyclic aromatic hydrocarbons in meat products by liquid chromatography. J Agric Food Chem. 1996. 44:2244–2251.
Article10. Dennis MJ, Massey RC, Cripps G, Venn I, Howarth N, Lee G. Factors affecting the polycyclic aromatic hydrocarbon content of cereals, fats and other food products. Food Addit Contam. 1991. 8:517–530.
Article11. Easton MPL, Luszinak D, Geest EV. Preliminary examination of contaminant loadings in farmed salmon, wild salmon and commercial salmon feed. Chemosphere. 2002. 46:1053–1074.
Article12. Fouchecourt MO, Berny P, Riviere JL. Bioavailability of PCBs to male laboratory rats maintained on litters of contaminated soils: PCB burden and induction of alkoxyresorufin O-dealkylase activities in liver and lung. Arch Environ Contam Toxicol. 1998. 35:680–687.13. Godschalk RWL, Verner ITM, Kriek E, Floot B, Schilderman PAEL, Moonen EJC, Kleinjans JCS, van Schooten FJ. Comparison of 32P-postlabeling and HPLCFD analysis of DNA adduct in rat acutely exposed to benzo(a)pyrene. Chem Biol Interact. 1997. 104:41–54.
Article14. Gräslund A, Jernström B. DNA-carcinogen interaction: covalent DNA-adducts of benzo(a)pyrene 7,8-dihydrodiol-9,10-epoxides studied by biochemical and biophysical techniques. Q Rev Biophys. 1989. 22:1–37.
Article15. Gravato C, Santos MA. Juvenile sea bass liver P450, EROD induction, and erythrocytic genotoxic responses to PAH and PAH-like compounds. Ecotoxicol Environ Saf. 2002. 51:115–127.
Article16. Gündel J, Schaller KH, Angerer J. Occupational exposure to polycyclic aromatic hydrocarbons in a fireproof stone producing plant: biological monitoring of 1-hydroxypyrene, 1-, 2-, 3- and 4-hydroxyphenanthrene, 3-hydroxybenz(a)anthracene and 3-hydroxybenzo(a)pyrene. Int Arch Occup Environ Health. 2000. 73:270–274.
Article17. Hansen AM, Poulsen OM, Christensen JM. Determination of 1-hydroxypyrene in human urine by HPLC. J Anal Toxicol. 1993. 17:38–41.18. IARC. IARC Monographs on the Evaluation of Carcinogenic Risk to Human. 1983. 32. Lyon: IARC;211.19. Islam GA, Greibrok T, Harvey RG, Øverebø S. HPLC analysis of benzo[a]pyrene-albumin adducts in benzo[a]pyrene exposed rats. Detection of cis-tetrols arising from hydrolysis of adducts of anti- and syn-BPDE III with proteins. Chem Biol Interact. 1999. 123:133–148.20. Jacob J, Grimmer G. Metabolism and excretion of polycyclic aromatic hydrocarbons in rat and in human. Cent Eur J Public Health. 1996. 4:33–39.21. Jongeneelen FJ, Akker WVD, Bos RP, Anzion RBM, Theuws JLG, Roelofs HMJ, Henderson PTH. 1-OH-pyrene as an indicator of mutagenicity of coal tar after activation with human liver preparation. Mutat Res. 1988. 204:195–201.
Article22. Jongeneelen FJ, Anizon RBM, Scheepers PTJ. 1-hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann Occup Hyg. 1988. 32:35–43.23. Jongeneelen FJ. Benchmark guideline for urinary 1- as biomarker of occupational exposure to polycyclic aromatic hydrocarbon. Ann Occup Hyg. 2001. 45:3–13.
Article24. Kazerouni N, Shinha R, Hsu CH, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001. 39:423–436.
Article25. Keimig SD, Kiby KW, Morgna PP. Identification of 1-hydroxypyrene as a major metabolite of pyrene in pig urine. Xenobiotica. 1983. 13:415–420.
Article26. Marczynski B, Rihs HP, Rossbach B, Holzer J, Angerer J, Scherenberg M, Hoffmann G, Bruning T, Wilhelm M. Analysis of 8-oxo-7,8-dihydro-2'-deoxyguanosine and DNA strand breaks in white blood cells of occupationally exposed workers: comparison with ambient monitoring, urinary metabolites and enzyme polymorphisms. Carcinogenesis. 2002. 23:273–281.
Article27. Mitchell CE, Tu KW. Distribution, retention, and elimination of pyrene in rats after inhalation. J Toxicol Environ Health. 1979. 5:1171–1179.
Article28. Moorthy B, Sriram P, Randerath K. Chemical structures and time-dependent effects of polycyclic aromatic hydrocarbon-type inducers on rat liver cytochrome P450, DNA adducts, and I-compounds. Fundamental Appl Toxicol. 1994. 22:549–560.
Article29. Nilsen A, Trønnes T, Westerholm R, Rannug U, Nilsen OG, Helleberg H, Kautiainen A, Hedenskog M, Törnqvist M. Short term exposure of rodent to disel exhausts; usefulness for studies of genotoxic and immunotoxic effect. Chem Biol Interact. 1999. 118:19–38.
Article30. Nisbet ICT, LaGoy PK. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons. Regul Toxicol Pharmacol. 1992. 16:290–300.31. Ovrebo S, Haugen A, Fjeldstad PE, Hemminkj K, Szyfter K. Biological monitoring of exposure to polycyclic aromatic hydrocarbon in an electrode paste plant. J Occup Med. 1994. 36:303–310.
Article32. Park SS, Kim YJ, Kang CH. Atomospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos Environ. 2002. 36:2917–2924.33. Parvanello S, Zanesi N, Levis AG. BaP metabolism and DNA -adduct formation in cultured human lymphocytes treated in vitro with BaP and BaP-7,8-dihydrodiol. ATLA. 1992. 20:126–137.34. Pavanello S, Favretto D, Brugnone F, Mastrangelo G, Dal Pra G, Clonfero E. HPLC/fluorescence determination of anti-BPDE-DNA adducts in mononuclear white blood cells from PAH-exposed humans. Carcinogenesis. 1999. 20:431–435.
Article35. Pohl RJ, Fouts JR. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fraction. Anal Biochem. 1980. 107:150–155.
Article36. Rojas M, Alexandrov K, von Schooten FS, Hillebrand M, Kriek E, Barstch H. Validation of a new fluorimetric assay for benzo[a]pyrene diolepoxide-DNA adducts in human white blood cells: comparisons with 32P-postlabeling and ELISA. Carcinogenesis. 1995. 15:557–560.
Article37. Roos PH, Tschirbs S, Pfeifer F, Welge P, Hack A, Wilhelm M, Bolt HM. Risk potentials for humans of original and remediated PAH-contaminated soils: application of biomarkers of effect. Toxicology. 2004. 205:181–194.
Article38. Roos PH, van Afferden M, Strotkamp D, Tappe D, Pfeifer F, Hanstein WG. Liver microsomal levels of cytochrome P450IA1 as biomarker for exposure and bioavailability of soil-bound polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol. 1996. 30:107–113.
Article39. Saunders CR, Ramesh A, Shockley DC. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol Lett. 2002. 129:33–45.
Article40. Sjögren M, Ehrenbrg L, Rannug U. Relevance of different biological assays in assessing initiating and promoting properties of polycyclic aromatic hydrocarbons with respect to carcinogenic potency. Mutat Res. 1996. 358:97–112.
Article41. WHO. WHO Food Additives Series 28. Evaluation of Certain Food Additives and Contaminants; Benzo(a)pyrene. 1998. Geneva: WHO;27–29.42. Willett KL, Wassenberg D, Lienesch L, Reichert W, Di Giulio RT. In vivo and in vitro inhibition of CYP1A-dependent activity in Fundulus heteroclitus by the polynuclear aromatic hydrocarbon fluoranthene. Toxicol Appl Pharmacol. 2001. 177:264–271.
Article43. Withey JR, Law FC, Endrenyi L. Pharmacokinetics and bioavailability of pyrene in the rat. J Toxicol Environ Health. 1991. 32:429–447.
Article44. Yamaguchi K, Near R, Shneider A, Cui H, Ju ST, Sherr DH. Fluoranthecene-induced apoptosis in murine T cell hybridomas is independent of the aromatic hydrocarbon receptor. Toxicol Appl Pharmacol. 1996. 139:144–152.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of ganoderma incidum on mouse pulmonary adenoma induced by benzo(a)pyrene
- DNA-Protein Crosslinks Formation by Benzo[a]pyrene and the Metabolites in BALB/3T3 cells
- Health Risk of Organic Pollutants in the Suspended Particulates in a Traffic Area of Seoul
- Inhibitory effects of biochanin A on mouse lung tumor induced by benzo(a)pyrene
- Studies on Benzo(a)pyrene of the Suspended Particulate in Atmosphere of Seoul City