J Vet Sci.
2008 Dec;9(4):387-393. 10.4142/jvs.2008.9.4.387.
Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs
- Affiliations
-
- 1Department of Veterinary Surgery, Seoul National University, Seoul 151-742, Korea. ohkweon@snu.ac.kr
- 2Laboratories of Stem Cell and Tumor Biology, Department of Veterinary Public Health, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. kangpub@snu.ac.kr
- 3Department of Biodesign, Institute of Biomaterials & Bioengineering, Tokyo Medical & Dental University, Tokyo, Japan.
- 4Biomaterials Center, National Institute for Materials Science, Tsukuba, Japan.
- KMID: 1104914
- DOI: http://doi.org/10.4142/jvs.2008.9.4.387
Abstract
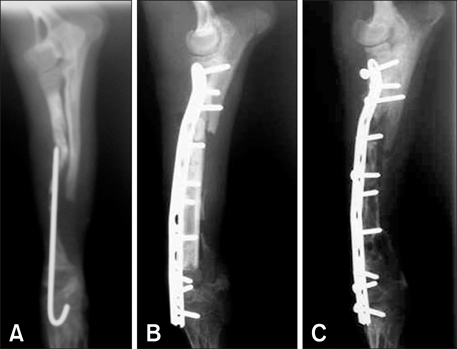
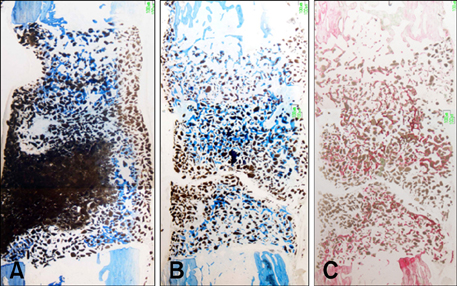
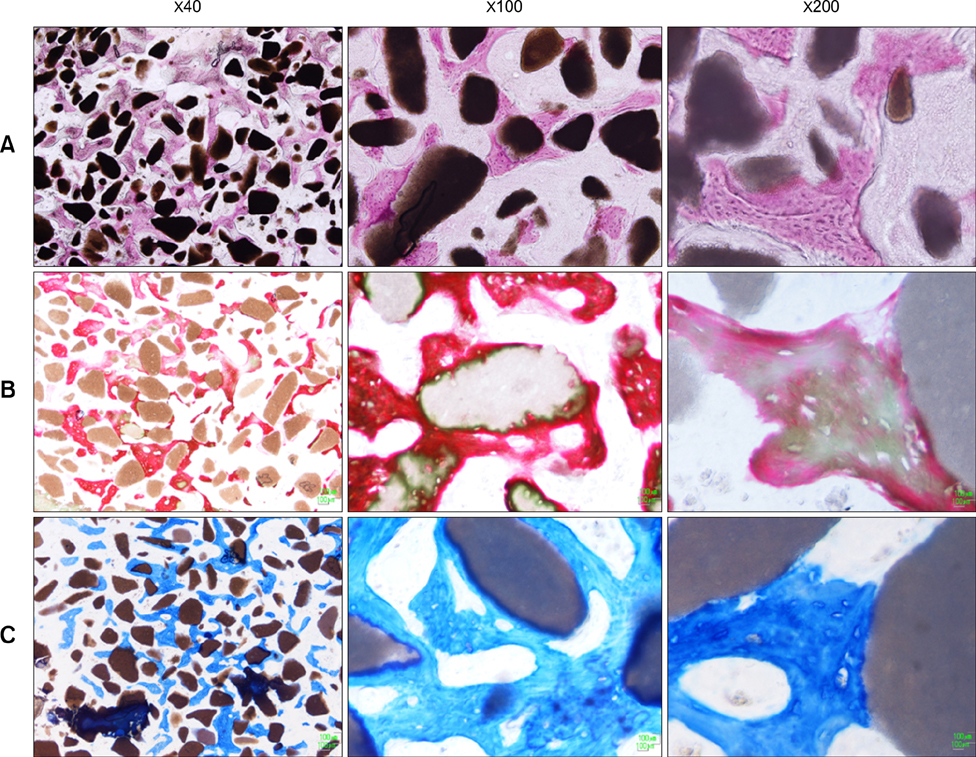
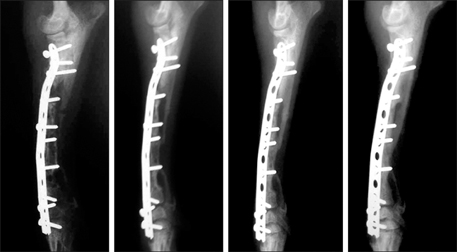
- This study was performed to evaluate the osteogenic effect of allogenic canine umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) mixed with beta-tricalcium phosphate (beta-TCP) in orthotopic implantation. Seven hundred milligrams of beta-TCP mixed with 1 x 10(6) UCB-MSCs diluted with 0.5 ml of saline (group CM) and mixed with the same volume of saline as control (group C) were implanted into a 1.5 cm diaphyseal defect and wrapped with PLGC membrane in the radius of Beagle dogs. Radiographs of the antebrachium were made after surgery. The implants were harvested 12 weeks after implantation and specimens were stained with H&E, toluidine blue and Villanueva-Goldner stains for histological examination and histomorphometric analysis of new bone formation. Additionally, UCB-MSCs were applied to a dog with non-union fracture. Radiographically, continuity between implant and host bone was evident at only one of six interfaces in group C by 12 weeks, but in three of six interfaces in group CM. Radiolucency was found only near the bone end in group C at 12 weeks after implantation, but in the entire graft in group CM. Histologically, bone formation was observed around beta-TCP in longitudinal sections of implant in both groups. Histomorphometric analysis revealed significantly increased new bone formation in group CM at 12 weeks after implantation (p < 0.05). When applied to the non-union fracture, fracture healing was identified by 6 weeks after injection of UCB-MSCs. The present study indicates that a mixture of UCB-MSCs and beta-TCP is a promising osteogenic material for repairing bone defects.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effect of serum-derived albumin scaffold and canine adipose tissue-derived mesenchymal stem cells on osteogenesis in canine segmental bone defect model
Daeyoung Yoon, Byung-Jae Kang, Yongsun Kim, Seung Hoon Lee, Daeun Rhew, Wan Hee Kim, Oh-Kyeong Kweon
J Vet Sci. 2015;16(4):397-404. doi: 10.4142/jvs.2015.16.4.397.
Reference
-
1. Arinzeh TL, Peter SJ, Archambault MP, Van Den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003. 85:1927–1935.
Article2. Arinzeh TL, Tran T, Mcalary J, Daculsi G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials. 2005. 26:3631–3638.
Article3. Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998. 80:985–996.
Article4. Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006. 24:679–685.
Article5. Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008. 3:e1886.
Article6. Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials. 1998. 19:1473–1478.
Article7. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–3843.
Article8. Fossum TW, Hedlund CS, Johnson AL, Schulz KS, Seim HB, Willard MD, Bahr A, Carroll GL. Small Animal Surgery. 2007. 3rd ed. St. Louis: Mosby;930–1014.9. Heise U, Osborn JF, Duwe F. Hydroxyapatite ceramic as a bone substitute. Int Orthop. 1990. 14:329–338.
Article10. Jäger M, Sager M, Knipper A, Degistirici O, Fischer J, Kögler G, Wernet P, Krauspe R. In vivo and in vitro bone regeneration from cord blood derived mesenchymal stem cells. Orthopade. 2004. 33:1361–1372.11. Johnson AL, Stein LE. Morphologic comparison of healing patterns in ethylene oxide-sterilized cortical allografts and untreated cortical autografts in the dog. Am J Vet Res. 1998. 49:101–105.12. Kadiyala S, Jaiswal N, Bruder SP. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997. 3:173–185.
Article13. Kadiyala S, Young RG, Thiede MA, Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997. 6:125–134.
Article14. Kasten P, Vogel J, Luginbühl R, Niemeyer P, Tonak M, Lorenz H, Helbig L, Weiss S, Fellenberg J, Leo A, Simank HG, Richter W. Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials. 2005. 26:5879–5889.
Article15. Kikuchi M, Koyama Y, Yamada T, Imamura Y, Okada T, Shirahama N, Akita K, Takakuda K, Tanaka J. Development of guided bone regeneration membrane composed of beta-tricalcium phosphate and poly (L-lactide-co-glycolide-co-epsilon-caprolactone) composites. Biomaterials. 2004. 25:5979–5986.
Article16. Kitaura H, Nagata N, Fujimura Y, Hotokezaka H, Yoshida N, Nakayama K. Effect of IL-12 on TNF-alpha-mediated osteoclast formation in bone marrow cells: apoptosis mediated by Fas/Fas ligand interaction. J Immunol. 2000. 169:4732–4738.
Article17. Koepp HE, Schorlemmer S, Kessler S, Brenner RE, Claes L, Günther KP, Ignatius AA. Biocompatibility and osseointegration of beta-TCP: histomorphological and biomechanical studies in a weight-bearing sheep model. J Biomed Mater Res B Appl Biomater. 2004. 70:209–217.18. Kraus KH, Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet Surg. 2006. 35:232–242.
Article19. Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004. 15:49–60.
Article20. Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun. 2007. 356:836–842.
Article21. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007. 8:275–282.
Article22. Lu J, Descamps M, Dejou J, Koubi G, Hardouin P, Lemaitre J, Proust JP. The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res. 2002. 63:408–412.
Article23. Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005. 95:827–839.
Article24. Olmstead ML. Small Animal Orthopedics. 1995. St. Louis: Mosby;150.25. Oonishi H. Orthopaedic applications of hydroxyapatite. Biomaterials. 1991. 12:171–178.
Article26. Penolazzi L, Lambertini E, Tavanti E, Torreggiani E, Vesce F, Gambari R, Piva R. Evaluation of chemokine and cytokine profiles in osteoblast progenitors from umbilical cord blood stem cells by BIO-PLEX technology. Cell Biol Int. 2008. 32:320–325.
Article27. Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004. 18:893–908.
Article28. Sartoris DJ, Holmes RE, Resnick D. Coralline hydroxyapatite bone graft substitutes: radiographic evaluation. J Foot Surg. 1992. 31:301–313.29. Steffen T, Stoll T, Arvinte T, Schenk RK. Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery. Eur Spine J. 2001. 10:Suppl 2. S132–S140.
Article30. Tang CH, Yang RS, Chen YF, Fu WM. Basic fibroblast growth factor stimulates fibronectin expression through phospholipase C gamma, protein kinase C alpha, c-Src, NF-kappaB, and p300 pathway in osteoblasts. J Cell Physiol. 2007. 211:45–55.
Article31. Tseng SS, Lee MA, Reddi AH. Nonunions and the potential of stem cells in fracture-healing. J Bone Joint Surg Am. 2008. 90:Suppl 1. 92–98.
Article32. van Hemert WL, Willems K, Anderson PG, van Heerwaarden RJ, Wymenga AB. Tricalcium phosphate granules or rigid wedge preforms in open wedge high tibial osteotomy: a radiological study with a new evaluation system. Knee. 2004. 11:451–456.
Article33. Walsh WR, Chapman-Sheath PJ, Cain S, Debes J, Bruce WJM, Svehla MJ, Gillies RM. A resorbable porous ceramic composite bone graft substitute in a rabbit metaphyseal defect model. J Orthop Res. 2003. 21:655–661.
Article34. Wang J, Asou Y, Sekiya I, Sotome S, Orii H, Shinomiya K. Enhancement of tissue engineered bone formation by a low pressure system improving cell seeding and medium perfusion into a porous scaffold. Biomaterials. 2006. 27:2738–2746.
Article35. Xu LX, Kukita T, Kukita A, Otsuka T, Niho Y, Iijima T. Interleukin-10 selectively inhibits osteoclastogenesis by inhibiting differentiation of osteoclast progenitors into preosteoclast-like cells in rat bone marrow culture system. J Cell Physiol. 1995. 165:624–629.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of serum-derived albumin scaffold and canine adipose tissue-derived mesenchymal stem cells on osteogenesis in canine segmental bone defect model
- Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells
- Osteogenic potential of mesenchymal cells derived from canine umbilical cord matrix co-cultured with platelet-rich plasma and demineralized bone matrix