J Vet Sci.
2008 Dec;9(4):367-373. 10.4142/jvs.2008.9.4.367.
In vitro and in vivo gene therapy with CMV vector-mediated presumed dog beta-nerve growth factor in pyridoxine-induced neuropathy dogs
- Affiliations
-
- 1Department of Veterinary Internal Medicine, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. hyyoun@snu.ac.kr
- 2Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 3Center for Laboratory Animal Science, College of Medicine, Hanyang University, Seoul 133-791, Korea.
- KMID: 1104911
- DOI: http://doi.org/10.4142/jvs.2008.9.4.367
Abstract
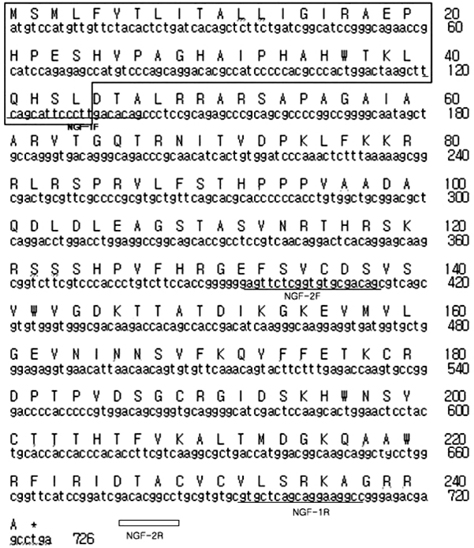
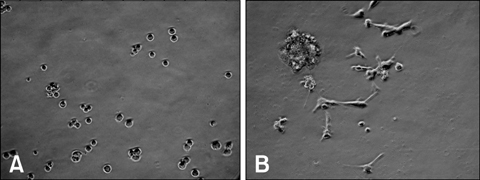
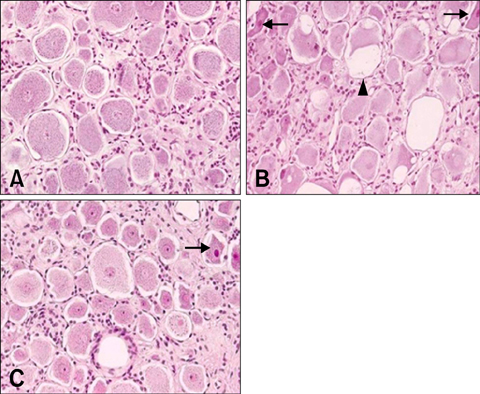
- Due to the therapeutic potential of gene therapy for neuronal injury, many studies of neurotrophic factors, vectors, and animal models have been performed. The presumed dog beta-nerve growth factor (pdbeta-NGF) was generated and cloned and its expression was confirmed in CHO cells. The recombinant pdbeta-NGF protein reacted with a human beta-NGF antibody and showed bioactivity in PC12 cells. The pdbeta-NGF was shown to have similar bioactivity to the dog beta-NGF. The recombinant pdbeta-NGF plasmid was administrated into the intrathecal space in the gene therapy group. Twenty-four hours after the vector inoculation, the gene therapy group and the positive control group were intoxicated with excess pyridoxine for seven days. Each morning throughout the test period, the dogs' body weight was taken and postural reaction assessments were made. Electrophysiological recordings were performed twice, once before the experiment and once after the test period. After the experimental period, histological analysis was performed. Dogs in the gene therapy group had no weight change and were normal in postural reaction assessments. Electrophysiological recordings were also normal for the gene therapy group. Histological analysis showed that neither the axons nor the myelin of the dorsal funiculus of L(4) were severely damaged in the gene therapy group. In addition, the dorsal root ganglia of L(4) and the peripheral nerves (sciatic nerve) did not experience severe degenerative changes in the gene therapy group. This study is the first to show the protective effect of NGF gene therapy in a dog model.
Keyword
MeSH Terms
-
Amino Acid Sequence
Animals
Base Sequence
CHO Cells
Central Nervous System Diseases/chemically induced/therapy/*veterinary
Cloning, Molecular
Cricetinae
Cricetulus
Cytomegalovirus
Dog Diseases/*chemically induced/therapy
Dogs
Female
Gene Therapy/*veterinary
Genetic Vectors
Male
Molecular Sequence Data
Nerve Growth Factor/genetics/*metabolism/*therapeutic use
Pyridoxine/*toxicity
Figure
Reference
-
1. Anderson DM, Hall LL, Ayyalapu AR, Irion VR, Nantz MH, Hecker JG. Stability of mRNA/cationic lipid lipoplexes in human and rat cerebrospinal fluid: methods and evidence for nonviral mRNA gene delivery to the central nervous system. Hum Gene Ther. 2003. 14:191–202.
Article2. Angeletti RH, Bradshaw RA. Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc Natl Acad Sci USA. 1971. 68:2417–2420.
Article3. Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999. 41:Suppl 1. 27–34.
Article4. Callizot N, Warter JM, Poindron P. Pyridoxine-induced neuropathy in rats: a sensory neuropathy that responds to 4-methylcatechol. Neurobiol Dis. 2001. 8:626–635.
Article5. Cao YJ, Shibata T, Rainov NG. Liposome-mediated transfer of the bcl-2 gene results in neuroprotection after in vivo transient focal cerebral ischemia in an animal model. Gene Ther. 2002. 9:415–419.
Article6. Chattopadhyay M, Goss J, Lacomis D, Goins WC, Glorioso JC, Mata M, Fink DJ. Protective effect of HSV-mediated gene transfer of nerve growth factor in pyridoxine neuropathy demonstrates functional activity of trkA receptors in large sensory neurons of adult animals. Eur J Neurosci. 2003. 17:732–740.
Article7. Chattopadhyay M, Wolfe D, Huang S, Goss J, Glorioso JC, Mata M, Fink DJ. In vivo gene therapy for pyridoxine-induced neuropathy by herpes simplex virus-mediated gene transfer of neurotrophin-3. Ann Neurol. 2002. 51:19–27.
Article8. Chattopadhyay M, Wolfe D, Mata M, Huang S, Glorioso JC, Fink DJ. Long-term neuroprotection achieved with latency-associated promoter-driven herpes simplex virus gene transfer to the peripheral nervous system. Mol Ther. 2005. 12:307–313.
Article9. Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol. 1994. 35:304–311.
Article10. Chung JY, Choi JH, Hwang CY, Youn HY. Pyridoxine induced neuropathy by subcutaneous administration in dogs. J Vet Sci. 2008. 9:127–131.
Article11. Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Rev. 1998. 27:1–39.
Article12. Hoover DM, Carlton WW. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) in beagle dogs. I. Clinical disease. Vet Pathol. 1981. 18:745–756.
Article13. Hoover DM, Carlton WW. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) in beagle dogs. II. Pathology. Vet Pathol. 1981. 18:757–768.
Article14. Hopkins AP, Gilliatt RW. Motor and sensory nerve conduction velocity in the baboon: normal values and changes during acrylamide neuropathy. J Neurol Neurosurg Psychiatry. 1971. 34:415–426.
Article15. Iwane M, Kitamura Y, Kaisho Y, Yoshimura K, Shintani A, Sasada R, Nakagawa S, Kawahara K, Nakahama K, Kakinuma A. Production, purification and characterization of biologically active recombinant human nerve growth factor. Biochem Biophys Res Commun. 1990. 171:116–122.
Article16. Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968. 48:534–569.
Article17. Mata M, Chattopadhyay M, Fink DJ. Gene therapy for the treatment of sensory neuropathy. Expert Opin Biol Ther. 2006. 6:499–507.
Article18. Sims MH, Selcer RR. Occurrence and evaluation of a reflex-evoked muscle potential (H reflex) in the normal dog. Am J Vet Res. 1981. 42:975–983.19. Ullrich A, Gray A, Berman C, Dull TJ. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983. 303:821–825.
Article20. Yang K, Clifton GL, Hayes RL. Gene therapy for central nervous system injury: the use of cationic liposomes: an invited review. J Neurotrauma. 1997. 14:281–297.
Article21. Zou LL, Huang L, Hayes RL, Black C, Qiu YH, Perez-Polo JR, Le W, Clifton GL, Yang K. Liposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applications. Gene Ther. 1999. 6:994–1005.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adenovirus-mediated Suicide Gene Therapy for Experimental Prostate Cancers with in Vivo Tumor Transduction Using the Herpes Simplex Virus Thymidine Kinase Gene/Acyclovir System
- Pyridoxine induced neuropathy by subcutaneous administration in dogs
- Prevention of Diabetes Using Adenoviral Mediated Hepatocyte Growth Factor Gene Transfer in Mice
- Stromelysin Gene Expression in Rat Eye Mediated by the Adenovirus Vector
- Comparative Study in DNA-mediated Vaccination Efficaency Among the Plasmids with Different Promoters