J Vet Sci.
2007 Mar;8(1):57-64. 10.4142/jvs.2007.8.1.57.
Development of a monoclonal antibody-based co-agglutination test to detect enterotoxigenic Escherichia coli isolated from diarrheic neonatal calves
- Affiliations
-
- 1Intas Biopharmaceuticals Ltd., Plot No. 423/P/A/GIDC, Sarkhej-Bavla Highway, Moraiya, Ahmedabad-382 210, Gujarat, India. brajesh.varshney@intasbiopharma.co.in, brij022002@yahoo.co.in
- 2R&D, Biotechnology, National Dairy Development Board, Anand-388 001, Gujarat, India.
- KMID: 1104237
- DOI: http://doi.org/10.4142/jvs.2007.8.1.57
Abstract
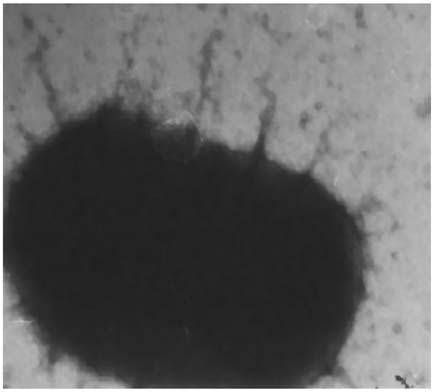
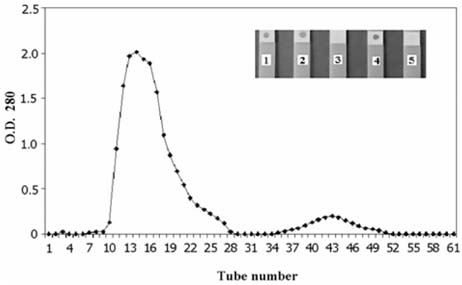
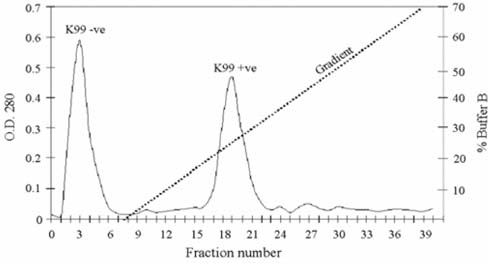
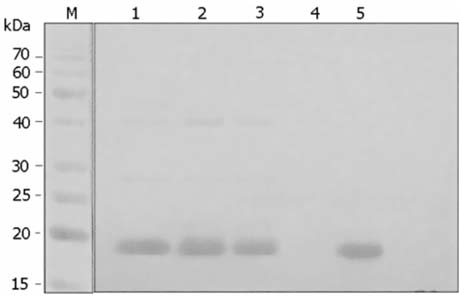
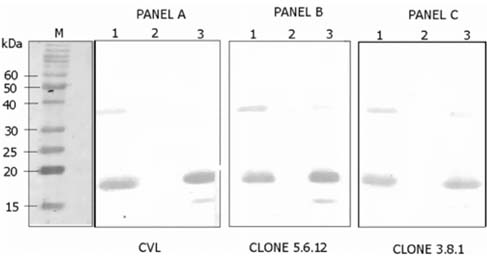
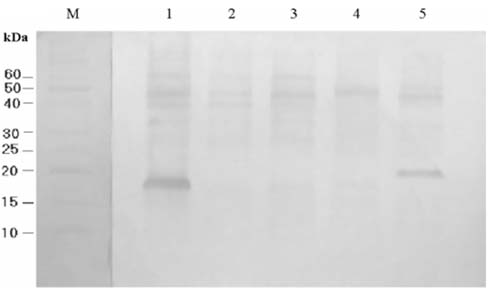
- Escherichia coli (E. coli) strains were collected from young diarrheic calves in farms and field. Strains that expressed the K99 (F5) antigen were identified by agglutination tests using reference antibodies to K99 antigen and electron microscopy. The K99 antigen from a selected field strain (SAR-14) was heat-extracted and fractionated on a Sepharose CL-4B column. Further purification was carried out by sodium deoxycholate treatment and/or ion-exchange chromatography. Monoclonal antibodies to purified K99 antigen were produced by the hybridoma technique, and a specific clone, NEK99-5.6.12, was selected for propagation in tissue culture. The antibodies, thus obtained, were affinity-purified, characterized and coated onto Giemsastained Cowan-I strain of Staphylococcus aureus (S. aureus). The antibody-coated S. aureus were used in a coagglutination test to detect K99+ E. coli isolated from feces of diarrheic calves. The specificity of the test was validated against reference monoclonal antibodies used in co-agglutination tests, as well as in ELISA. Specificity of the monoclonal antibodies was also tested against various Gram negative bacteria. The developed antibodies specifically detected purified K99 antigen in immunoblots, as well as K99+ E. coli in ELISA and co-agglutination tests. The co-agglutination test was specific and convenient for large-scale screening of K99+ E. coli isolates.
MeSH Terms
-
Agglutination Tests/methods/*veterinary
Animals
*Animals, Newborn
Antibodies, Monoclonal/*immunology
Antigens, Surface/immunology/isolation & purification
Bacterial Toxins/immunology/isolation & purification
Cattle
Cattle Diseases/*immunology/*microbiology
Chromatography, Gel/veterinary
Chromatography, Ion Exchange/veterinary
Chromatography, Liquid/veterinary
Diarrhea/immunology/*veterinary
Electrophoresis, Polyacrylamide Gel/veterinary
Enzyme-Linked Immunosorbent Assay/veterinary
Escherichia coli/*immunology
Escherichia coli Infections/immunology/*veterinary
Immunoblotting/veterinary
Staphylococcus aureus
Figure
Reference
-
1. Altmann K, Pyliotis NA, Mukkur TKS. A new method for the extraction and purification of K99 pili from enterotoxigenic Escherichia coli and their characterization. Biochem J. 1982. 201:505–513.
Article2. Batra HV, Chand P, Thillaikoothan P, Talwar GP. Coagglutination test with coloured Staphylococcus aureus for detection of brucella antigens in cattle brucellosis. Vet Rec. 1987. 121:65–66.
Article3. Boedeker EC. Vaccines for enterotoxigenic Escherichia coli: current status. Curr Opin Gastroenterol. 2005. 21:15–19.4. Chakraborty S, Deokule JS, Garg P, Bhattacharya SK, Nandy RK, Nair GB, Yamasaki S, Takeda Y, Ramamurthy T. Concomitant infection of enterotoxigenic Escherichia coli in an outbreak of cholera caused by Vibrio cholerae O1 and O139 in Ahmedabad, India. J Clin Microbiol. 2001. 39:3241–3246.
Article5. de Graaf FK, Klemm P, Gaastra W. Purification, characterization, and partial covalent structure of Escherichia coli adhesive antigen K99. Infect Immun. 1981. 33:877–883.
Article6. Guinee PAM, Veldkamp J, Jansen WH. Improved minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli. Infect Immun. 1977. 15:676–678.
Article7. Holley DL, Allen SD, Barnett BB. Enzyme-linked immunosorbent assay, using monoclonal antibody, to detect enterotoxic Escherichia coli K99 antigen in feces of dairy calves. Am J Vet Res. 1984. 45:2613–2616.8. Jay CM, Bhaskaran S, Rathore KS, Waghela SD. Enterotoxigenic K99+ Escherichia coli attachment to host cell receptors inhibited by recombinant pili protein. Vet Microbiol. 2004. 101:153–160.
Article9. Jiang ZD, Lowe B, Verenkar MP, Ashley D, Steffen R, Tornieporth N, von Sonnenburg F, Waiyaki P, DuPont HL. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J Infect Dis. 2002. 185:497–502.
Article10. Kang G, Ramakrishna BS, Daniel J, Mathan M, Mathan VI. Epidemiological and laboratory investigations of outbreaks of diarrhoea in rural South India: implications for control of disease. Epidemiol Infect. 2001. 127:107–112.
Article11. Korhonen TK, Nurmiaho EL, Ranta H, Eden CS. New method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980. 27:569–575.
Article12. Kumar A, Sharma VD, Thapliyal DC. Occurrence of enterotoxigenic Escherichia coli in cow calves and infants/children. Indian J Comp Microbiol Immunol Infect Dis. 1982. 3:174–177.13. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.
Article14. Lugtenberg B, Meijers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975. 58:254–258.
Article15. Micklem LR, Mc Cann MC, James K. The use of rat mixed-thymocyte culture-conditioned medium for hybridoma production, cloning and revival. J Immunol Methods. 1987. 104:81–86.
Article16. Mills KW, Phillips RM, Kelly BL, Baughman GL. Using enzyme-linked immunosorbent assay to detect Escherichia coli K88 pili antigens from clinical isolates. Am J Vet Res. 1982. 43:365–367.17. Morris JA, Stevens AE, Sojka WJ. Preliminary characterization of cell-free K99 antigen isolated from Escherichia coli B41. J Gen Microbiol. 1977. 99:353–357.
Article18. Raybould TJG, Crouch CF, Acres SD. Monoclonal antibody passive hemagglutination and capture enzyme-linked immunosorbent assays for direct detection and quantitation of F41 and K99 fimbrial antigens in enterotoxigenic Escherichia coli. J Clin Microbiol. 1987. 25:278–284.
Article19. Rudin A, McConnell MM, Svennerholm AM. Monoclonal antibodies against enterotoxigenic Escherichia coli colonization factor antigen I (CFA/I) that cross-react immunologically with heterologous CFAs. Infect Immun. 1994. 62:4339–4346.
Article20. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 1989. 2nd ed. New York: Cold Spring Harbor Laboratory Press;18.47–18.67.21. Sussman M. Sussman M, editor. Escherichia coli in human and animal disease. The Virulence of Escherichia Coli: Review and Methods. 1985. London: Academic Press;7–45.22. Tacket CO, Maneval DR, Levine MM. Purification, morphology, and genetics of a new fimbrial putative colonization factor of enterotoxigenic Escherichia coli O159:H4. Infect Immun. 1987. 55:1063–1069.
Article23. Thorns CJ, Sawtell JA, Miller JA, Wood GW. Identification of the K99 fimbrial adhesin in commercial vaccines used against calf enteritis. Biologicals. 1990. 18:113–115.
Article24. Tsen HY, Jian LZ. Development and use of a multiplex PCR system for the rapid screening of heat labile toxin I, heat stable toxin II and shiga-like toxin I and II genes of Escherichia coli in water. J Appl Microbiol. 1998. 84:585–592.
Article25. Vazquez F, Gonzalez EA, Garabal JI, Blanco J. Fimbriae extracts from enterotoxigenic Escherichia coli strains of bovine and porcine origin with K99 and/or F41 antigens. Vet Microbiol. 1996. 48:231–241.26. Watterworth L, Topp E, Schraft H, Leung KT. Multiplex PCR-DNA probe assay for the detection of pathogenic Escherichia coli. J Microbiol Methods. 2005. 60:93–105.
Article27. Woodward MJ, Wray C. Nine DNA probes for detection of toxin and adhesin genes in Escherichia coli isolated from diarrhoeal disease in animals. Vet Microbiol. 1990. 25:55–65.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Virulence factors in Escherichia coli isolated from calves with diarrhea in Vietnam
- Prevalence of O-serogroups, virulence genes, and F18 antigenic variants in Escherichia coli isolated from weaned piglets with diarrhea in Korea during 2008–2016
- Inhibitory effects of several drugs to intestinal secretory stimulation of heat-labile enterotoxin produced by enterotoxigenic E. coli
- Survey of bovine norovirus infections from diarrheic calves in South Korea, 2015–2017
- Biological detection of enterotoxigenic E. coli