J Vet Sci.
2006 Mar;7(1):31-36. 10.4142/jvs.2006.7.1.31.
pH-dependent modulation of intracellular free magnesium ions with ionselective electrodes in papillary muscle of guinea pig
- Affiliations
-
- 1Department of Pharmacology & Toxicology, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea. kimjs@chonbuk.ac.kr
- 2Bio-Safety Research Institute, Chonbuk National University, Jeonju 561-756, Korea.
- 3Center for Healthcare Technology Development, Chonbuk National University, Jeonju 561-756, Korea.
- KMID: 1103550
- DOI: http://doi.org/10.4142/jvs.2006.7.1.31
Abstract
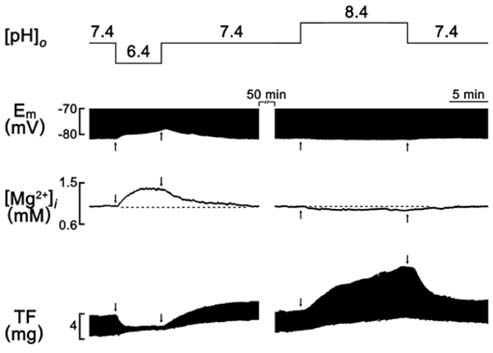
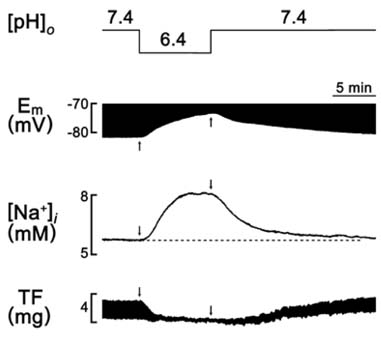
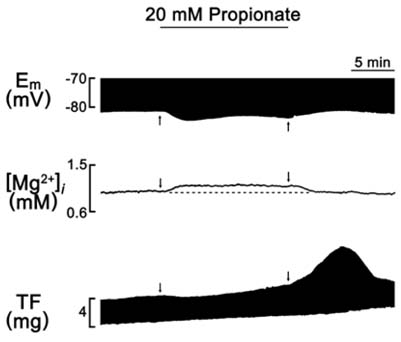
- A change in pH can alter the intracellular concentration of electrolytes such as intracellular Ca2+ and Na+ ([Na+]i) that are important for the cardiac function. For the determination of the role of pH in the cardiac magnesium homeostasis, the intracellular Mg2+ concentration ([Mg2+]i), membrane potential and contraction in the papillary muscle of guinea pigs using ion-selective electrodes changing extracellular pH ([pH]o) or intracellular pH ([pH]i) were measured in this study. A high CO2-induced low [pH]o causes a significant increase in the [Mg2+]i and [Na+]i, which was accompanied by a decrease in the membrane potential and twitch force. The high [pH]o had the opposite effect. These effects were reversible in both the beating and quiescent muscles. The low [pH]o-induced increase in [Mg2+]i occurred in the absence of [Mg2+]o. The [Mg2+]i was increased by the low [pH]i induced by propionate. The [Mg2+]i was increased by the low [pH]i induced by NH4Cl-prepulse and decreased by the recovery of [pH]i induced by the removal of NH4Cl. These results suggest that the pH can modulate [Mg2+]i with a reverse relationship in heart, probably by affecting the intracellular Mg2+ homeostasis, but not by Mg2+ transport across the sarcolemma.
MeSH Terms
Figure
Reference
-
1. Agus ZS, Morad M. Modulation of cardiac ion channels by magnesium. Annu Rev Physiol. 1991. 53:299–307.
Article2. Aomine M, Tatsukawa Y, Yamato T, Yamasaki S. Antiarrhythmic effects of magnesium on rat papillary muscle and guinea pig ventricular myocytes. Gen Pharmacol. 1999. 32:107–114.
Article3. Blatter LA, McGuigan JA. Intracellular pH regulation in ferret ventricular muscle. The role of Na-H exchange and the influence of metabolic substrates. Circ Res. 1991. 68:150–161.
Article4. Bountra C, Vaughan-Jones RD. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989. 418:163–187.
Article5. Borle AB, Bender C. Effects of pH on Ca2+i, Na+i, and pHi of MDCK cells: Na(+)-Ca2+ and Na(+)-H+ antiporter interactions. Am J Physiol. 1991. 261:C482–C489.
Article6. Buri A, Chen S, Fry CH, Illner H, Kickenwiez E, McGuigan JAS, Noble D, Powell T, Twist VW. The regulation of intracellular Mg2+ in guinea-pig heart, studied with Mg2+-selective microelectrodes and fluorochromes. Exp Physiol. 1993. 78:221–233.
Article7. Elliott AC, Smith GL, Allen DG. The metabolic consequences of an increase in the frequency of stimulation in isolated ferret hearts. J Physiol. 1994. 474:147–159.
Article8. Flatman PW. Mechanism of magnesium transport. Annu Rev Physiol. 1991. 53:259–271.9. Freudenrich CC, Murphy E, Levy LA, London RE, Lieberman M. Intracellular pH modulates cytosolic free magnesium in cultured chicken heart cells. Am J Physiol. 1992. 262:C1024–C1030.
Article10. Garlick PB, Radda GK, Seeley PJ. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J. 1979. 184:547–554.
Article11. Gunzel D, Durry S, Schlue WR. Intracellular alkalinization causes Mg2+ release from intracellular binding sites in leech Retzius neurons. Pflugers Arch. 1997. 435:65–73.12. Hall SK, Fry CH. Magnesium affects excitation, conduction, and contraction of isolated mammalian cardiac muscle. Am J Physiol. 1992. 263:H622–H633.
Article13. Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH. Contraction and intracellular Ca2+, Na+ and H+ during acidosis in rat ventricular myocytes. Am J Physiol. 1992. 262:C348–C357.14. Iseri LT, French JH. Magnesium: nature's physiologic calcium blocker. Am Heart J. 1984. 108:188–193.
Article15. Jung DW, Brierley GP. Magnesium transport by mitochondria. J Bioenerg Biomembr. 1994. 26:527–535.
Article16. Lee CO, Im WB, Sonn JK. Intracellular sodium ion activity: reliable measurement and stimulation-induced change in cardiac Purkinje fibers. Can J Physiol Pharmacol. 1987. 65:954–962.
Article17. Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol. 1999. 517:159–180.
Article18. Leonhard-Marek S, Gabel G, Martens H. Effects of short chain fatty acids and carbon dioxide on magnesium transport across sheep rumen epithelium. Exp Physiol. 1998. 83:155–164.
Article19. Leroy J. Nécessité du magnésium pour la croissence da la souris. omptes Rendus des Sceances de la Société de Biologie. 1926. 94:341.20. Li HY, Quamme GA. Effect of pH on intracellular free Mg2+ in isolated adult rat cardiomyocytes. Biochim Biophys Acta. 1994. 1222:164–170.
Article21. Maguire ME, Cowan JA. Magnesium chemistry and biochemistry. BioMetals. 2002. 15:203–210.22. Masumoto N, Tasaka K, Mizuki J, Miyake A, Tanizawa O. Regulation of intracellular Mg2+ by superoxide in amnion cells. Biochem Biophys Res Commun. 1992. 182:906–912.23. McGuigan JAS, Elder HY, Gunzel D, Schlue W-R. Magnesium Homeostasis in Heart; A Critical Reappraisal. J Clin Basic Cardiol. 2002. 5:5–22.24. Negulescu PA, Machen TE. Lowering extracellular sodium or pH raises intracellular calcium in gastric cells. J Membr Biol. 1990. 116:239–248.
Article25. Orchard CH, Cingolani HE. Acidosis and arrhythmias in cardiac muscle. Cardiovas Res. 1994. 28:1312–1319.
Article26. Rajdev S, Reynolds IJ. Calcium influx but not pH or ATP level mediates glutamate induced changes in intracellular magnesium in cortical neurons. J Neurophysiol. 1995. 74:942–949.
Article27. Romani AM, Maguire ME. Hormonal regulation of Mg2+ transport and homeostasis in eukaryotic cells. Biometals. 2002. 15:271–283.28. Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000. 294:1–26.29. Schweigel M, Vormann J, Martens H. Mechanisms of Mg(2+) transport in cultured ruminal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000. 278:G400–G408.30. Tavi P, Han C, Wecksto M. Intracellular acidosis modulates the stretch-induced changes in E-C coupling of the rat atrium. Acta Physiol Scand. 1999. 167:203–213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Vanadate on Cellular Ca2+ Movements in Guinea Pig Papillary Muscles
- Effects of Na+ and Ca2+ concentration in cardioplegic and reperfusion solutions on the intracellular Ca2+ of cardiac muscle cells
- Effects of ryanodine on the intracellular Na+ activity and tension and action potentials of rat and guinea pig cardiac ventricular muscles
- The effects of adriamycin on twitch force and membrane potential in an isolated Guinea-pig papillary muscle
- Regulation of Mg2+ release in guinea pig heart and isolated ventricular myocytes by alpha1-adrenergic stimulation