J Vet Sci.
2009 Jun;10(2):165-167. 10.4142/jvs.2009.10.2.165.
A novel beta-glucan produced by Paenibacillus polymyxa JB115 induces nitric oxide production in RAW264.7 macrophages
- Affiliations
-
- 1College of Veterinary Medicine, Kyungpook National University, Daegu 702-701, Korea. parksch@knu.ac.kr
- 2Bio Industry Center, Daegu Technopark, Daegu 704-701, Korea.
- 3Department of Food Science and Technology, Keimyung University, Daegu 704-701, Korea.
- KMID: 1102975
- DOI: http://doi.org/10.4142/jvs.2009.10.2.165
Abstract
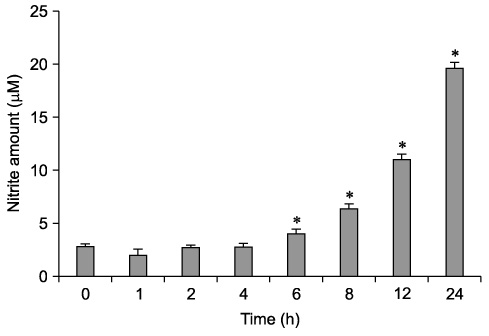
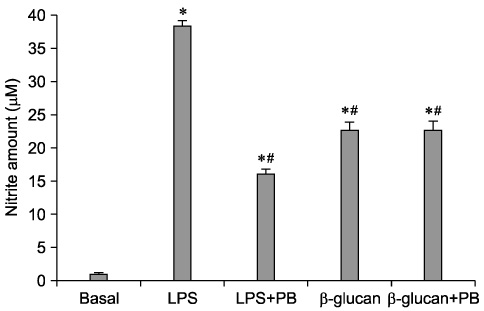
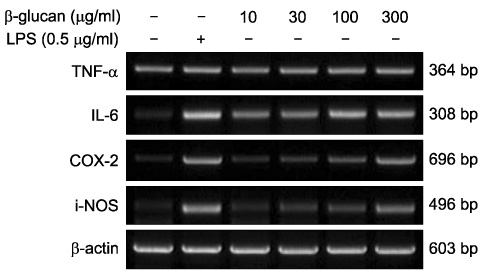
- The effect of extracellular beta-(1-->3), (1-->6)-glucan, produced by Paenibacillus polymyxa JB115, on nitric oxide (NO) production in RAW264.7 macrophages was investigated. beta-glucan induced the production of NO by RAW264.7 macrophages in a concentration- and time-dependent manner. Moreover, beta-glucan stimulation increased the mRNA expression of iNOS, COX-2 and IL-6 in RAW264.7 macrophages in a concentration-dependent manner.
Keyword
MeSH Terms
-
Animals
Bacillus/*metabolism
Cell Line
Cyclooxygenase 2/biosynthesis/genetics
Interleukin-6/biosynthesis/genetics
Lipopolysaccharides/pharmacology
Macrophages/*drug effects/enzymology/immunology
Mice
Nitric Oxide/*biosynthesis/immunology
Nitric Oxide Synthase Type II/biosynthesis/genetics/metabolism
RNA, Messenger/biosynthesis/genetics
Reverse Transcriptase Polymerase Chain Reaction
beta-Glucans/metabolism/*pharmacology
Figure
Reference
-
1. Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993. 150:5080–5085.2. Bohn JA, BeMiller JN. (1→3)-beta-D-glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr Polymers. 1995. 28:3–14.
Article3. Brown GD, Gordon S. Immune recognition of fungal β-glucans. Cell Microbiol. 2005. 7:471–479.
Article4. Chang ZQ, Oh BC, Rhee MH, Kim JC, Lee SP, Park SC. Polysaccharides isolated from Phellinus baumii stimulate murine splenocyte proliferation and inhibit the lipopolysaccharide-induced nitric oxide production in RAW264.7 murine macrophages. World J Microbiol Biotechnol. 2007. 23:723–727.
Article5. Dritz SS, Shi J, Kielian TL, Goodband RD, Nelssen JL, Tokach MD, Chengappa MM, Smith JE, Blecha F. Influence of dietary beta-glucan on growth performance, nonspecific immunity, and resistance to Streptococcus suis infection in weanling pigs. J Anim Sci. 1995. 73:3341–3350.
Article6. Johnson BA, Pitt BR, Davies P. Pulmonary arterial smooth muscle cells modulate cytokine- and LPS-induced cytotoxicity in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000. 278:L460–L468.
Article7. Jung HK, Hong JH, Park SC, Park BK, Nam DH, Kim SD. Production and physicochemical characterization of β-glucan produced by Paenibacillus polymyxa JB115. Biotechonol Bioprocess Eng. 2007. 12:713–719.
Article8. Kim GY, Choi GS, Lee SH, Park YM. Acidic polysaccharide isolated from Phellinus linteus enhances through the up-regulation of nitric oxide and tumor necrosis factor-alpha from peritoneal macrophages. J Ethnopharmacol. 2004. 95:69–76.
Article9. Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999. 84:253–256.
Article10. Lee JY, Kim JY, Lee YG, Rhee MH, Hong EK, Cho JY. Molecular mechanism of macrophage activation by exopolysaccharides from liquid culture of Lentinus edodes. J Microbiol Biotechnol. 2008. 18:355–364.11. Lee KY, Lee MH, Chang IY, Yoon SP, Lim DY, Jeon YJ. Macrophage activation by polysaccharide fraction isolated from Salicornia herbacea. J Ethnopharmacol. 2006. 103:372–378.
Article12. Li J, Li DF, Xing JJ, Cheng ZB, Lai CH. Effects of beta-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide. J Anim Sci. 2006. 84:2374–2381.
Article13. Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994. 120:227–237.
Article14. Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.15. Pacheco-Sánchez M, Boutin Y, Angers P, Gosselin A, Tweddell RJ. Inhibitory effect of CDP, a polysaccharide extracted from the mushroom Collybia dryophila, on nitric oxide synthase expression and nitric oxide production in macrophages. Eur J Pharmacol. 2007. 555:61–66.
Article16. Rice PJ, Adams EL, Ozment-Skelton T, Gonzalez AJ, Goldman MP, Lockhart BE, Barker LA, Breuel KF, Deponti WK, Kalbfleisch JH, Ensley HE, Brown GD, Gordon S, Williams DL. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther. 2005. 314:1079–1086.
Article17. Ross GD, Vetvicka V, Yan J, Xia Y, Vetvicková J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999. 42:61–74.
Article18. Sandula J, Kogan G, Kacurakova M, Machova E. Microbial (1→3)-β-D-glucans, their preparation, physicochemical characterization and immonomodulatory activity. Carbohydr Polym. 1999. 38:247–253.19. Schoenherr WD, Pollmann DS, Coalson JA. Titration of MacroGard-S on growth performance of nursery pigs. J Anim Sci. 1994. 72:Suppl 2. 57.20. Son CG, Shin JW, Cho JH, Cho CK, Yun CH, Chung W, Han SH. Macrophage activation and nitric oxide production by water soluble components of Hericium erinaceum. Int Immunopharmacol. 2006. 6:1363–1369.
Article21. Vogel SN, Marshall ST, Rosenstreich DL. Analysis of the effects of lipopolysaccharide on macrophages: differential phagocytic responses of C3H/HeN and C3H/HeJ macrophages in vitro. Infect Immun. 1979. 25:328–336.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maturation of bone marrow-derived dendritic cells by a novel beta-glucan purified from Paenibacillus polymyxa JB115
- Immunostimulatory Effects of beta-glucan Purified from Paenibacillus polymyxa JB115 on Mouse Splenocytes
- Immunomodulatory effect of beta-glucan derived from Saccharomyces cerevisiae strains
- Inhibitory Effects of Paeonia Suffruticosa on the Expression of Inducible Nitric Oxide Synthase Gene in RAW264.7 Macrophages
- Stimulation of macrophage function by S-antigen: production of nitric oxide