J Vet Sci.
2008 Mar;9(1):51-66. 10.4142/jvs.2008.9.1.51.
Use of flow cytometry to develop and characterize a set of monoclonal antibodies specific for rabbit leukocyte differentiation molecules
- Affiliations
-
- 1Department of Veterinary Microbiology and Pathology, College of Veterinary Medicine, Washington State University, Pullman, WA 99164-7040, USA. davisw@vetmed.wsu.edu
- KMID: 1102954
- DOI: http://doi.org/10.4142/jvs.2008.9.1.51
Abstract
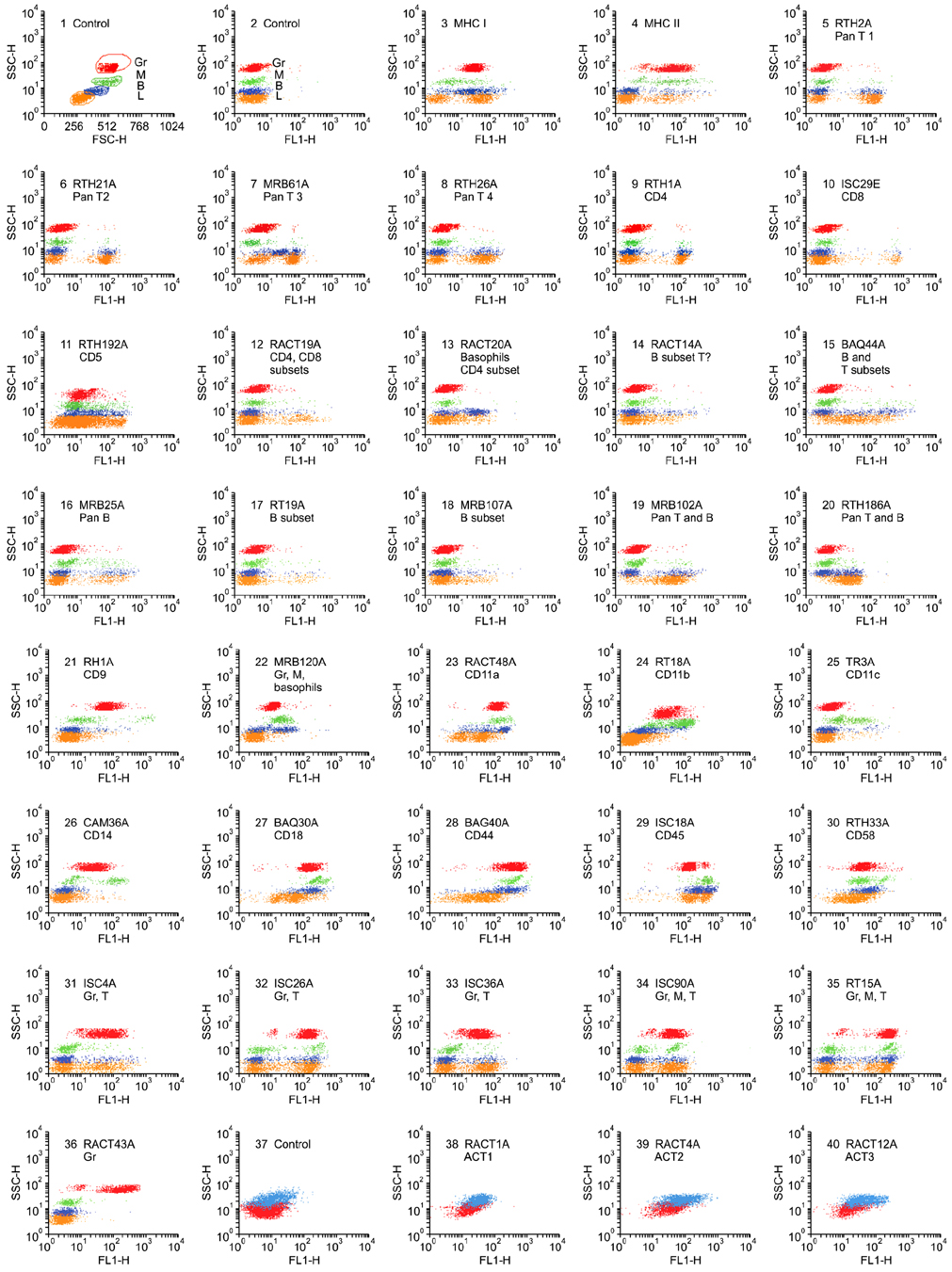
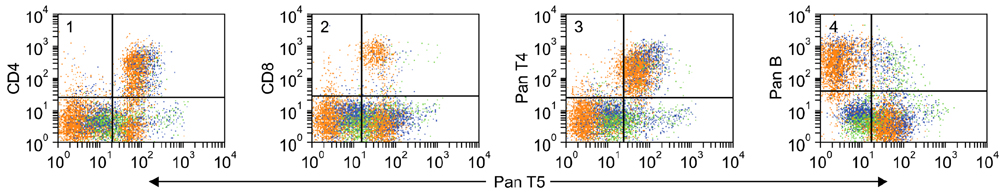
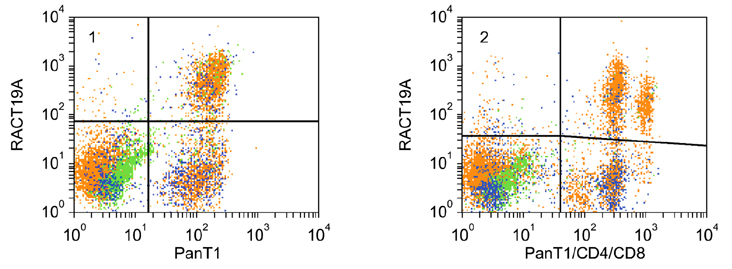
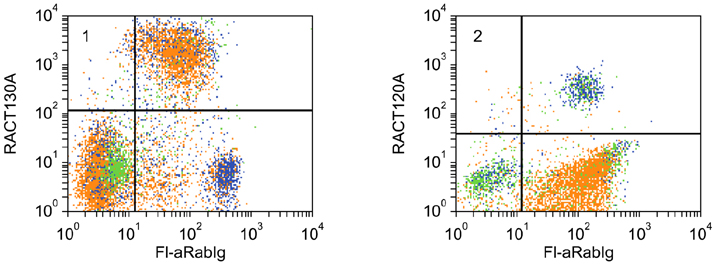
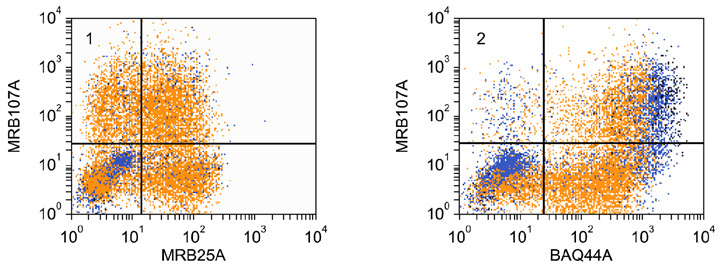
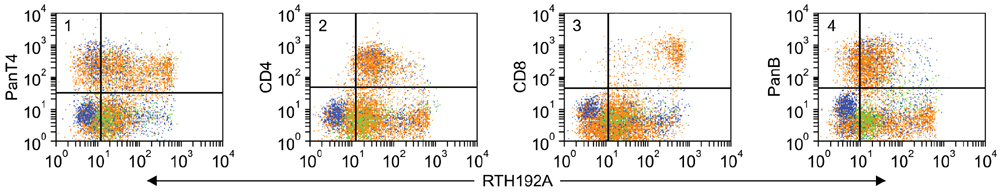
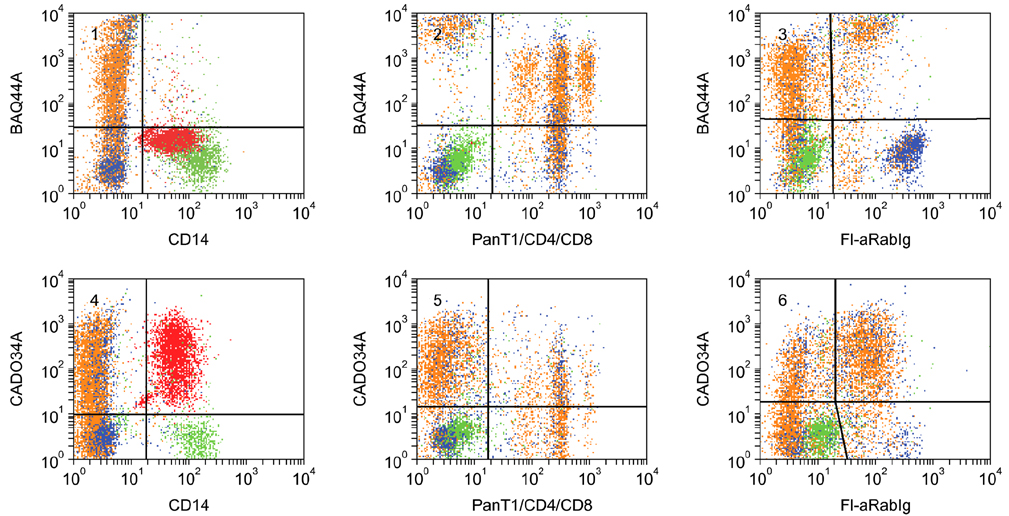
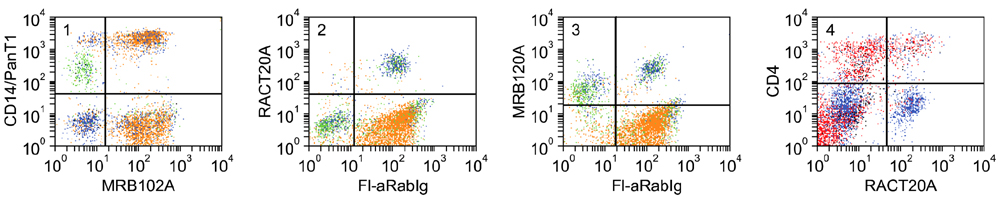
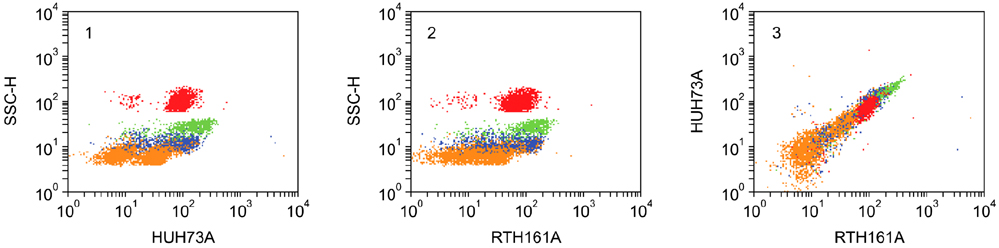
- Flow cytometry was used to identify and characterize monoclonal antibodies (mAbs) that react with rabbit leukocyte differentiation molecules (LDM). Screening sets of mAbs, developed against LDM in other species, for reactivity with rabbit LDM yielded 11 mAbs that recognize conserved epitopes on rabbit LDM orthologues and multiple mAbs that recognize epitopes expressed on the major histocompatibility class I or class II molecules. Screening of mAbs submitted to the Animal Homologues Section of the Eighth Human Leukocyte Differentiation Workshop yielded 7 additional mAbs. Screening of mAbs generated from mice immunized with leukocytes from rabbit thymus or spleen or concanavalin A activated peripheral blood and/or spleen lymphocytes has yielded 42 mAbs that recognize species restricted epitopes expressed on one or more lineages of leukocytes. Screening of the anti-rabbit mAbs against leukocytes from other species yielded one additional mAb. The studies show that screening of existing sets of mAbs for reactivity with rabbit LDM will not be productive and that a direct approach will be needed to develop mAbs for research in rabbits. The flow cytometric approach we developed to screen for mAbs of interest offers a way for individual laboratories to identify and characterize mAbs to LDM in rabbits and other species. A web-based program we developed provides a source of information that will facilitate analysis. It contains a searchable data base on known CD molecules and a data base on mAbs, known to react with LDM in one or more species of artiodactyla, equidae, carnivora, and or lagomorpha.
MeSH Terms
-
Animals
Antibodies, Monoclonal/*immunology
Antigens, Differentiation/*metabolism
B-Lymphocytes/cytology/metabolism
Basophils/cytology/metabolism
Epitopes/genetics/metabolism
*Flow Cytometry
Gene Expression Regulation
Granulocytes/cytology/metabolism
Leukocytes/immunology/*metabolism
Mice
Monocytes/cytology/metabolism
Rabbits
T-Lymphocytes/cytology/metabolism
Figure
Reference
-
1. Ababou A, Goyeneche J, Davis WC, Lévy D. Evidence for the expression of three different BoLa-class II molecules on the bovine BL-3 cell line: determination of a non-DR non-DQ gene product. J Leukoc Biol. 1994. 56:182–186.
Article2. Binns RM. The null/γδTCR+ T cell family in the pig. Vet Immunol Immunopathol. 1994. 43:69–77.
Article3. Binns RM, Duncan IA, Powis SJ, Hutchings A, Butcher GW. Subsets of null and γδ T-cell receptor+ T lymphocytes in the blood of young pigs identified by specific monoclonal antibodies. Immunology. 1992. 77:219–227.4. Brodersen R, Bijlsma F, Gori K, Jensen KT, Chen W, Dominguez J, Haverson K, Moore PF, Saalmuller A, Sachs D, Slierendrecht WJ, Stokes C, Vainio O, Zuckermann F, Aasted B. Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet Immunol Immunopathol. 1998. 64:1–13.
Article5. Bucy RP, Chen CH, Cooper MD. Analysis of γδ T cells in the chicken. Semin Immunol. 1991. 3:109–117.6. Cabana VG, Teodorescu M, Dray S. Identification of basophils as the cells bearing both allelic immunoglobulin allotypes among white blood cells from the peripheral blood of heterozygous rabbits. J Immunol. 1980. 124:2268–2280.7. Carr MM, Howard CJ, Sopp P, Manser JM, Parsons KR. Expression on porcine γδ lymphocytes of a phylogenetically conserved surface antigen previously restricted in expression to ruminant γδ T lymphocytes. Immunology. 1994. 81:36–40.8. Cobbold S, Metcalfe S. Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW). Tissue Antigens. 1994. 43:137–154.
Article9. Cooper MD, Chen C-LH, Bucy RP, Thompson CB. Avian T cell ontogeny. Adv Immunol. 1991. 50:87–117.
Article10. Davis WC, Brown WC, Hamilton MJ, Wyatt CR, Orden JA, Khalid AM, Naessens J. Analysis of monoclonal antibodies specific for the γδ TcR. Vet Immunol Immunopathol. 1996. 52:275–283.
Article11. Davis WC, Davis JE, Hamilton MJ. Davis WC, editor. Use of monoclonal antibodies and flow cytometry to cluster and analyze leukocyte differentiation molecules. Monoclonal Antibody Protocols. 1995. Totowa: Humana Press;149–167.
Article12. Davis WC, Drbal K, Mosaad AE, Elbagory AR, Tibary A, Barrington GM, Park YH, Hamilton MJ. Use of flow cytometry to identify monoclonal antibodies that recognize conserved epitopes on orthologous leukocyte differentiation antigens in goats, lamas, and rabbits. Vet Immunol Immunopathol. 2007. 119:123–130.
Article13. Davis WC, Hamilton MJ. Use of flow cytometry to characterize immunodeficiency syndromes in camelids. Small Rumin Res. 2006. 61:187–193.
Article14. Davis WC, Heirman LR, Hamilton MJ, Parish SM, Barrington GM, Loftis A, Rogers M. Flow cytometric analysis of an immunodeficiency disorder affecting juvenile llamas. Vet Immunol Immunopathol. 2000. 74:103–120.
Article15. Davis WC, Khalid AM, Hamilton MJ, Ahn JS, Park YH, Cantor GH. The use of crossreactive monoclonal antibodies to characterize the immune system of the water buffalo (Bubalus bubalus). J Vet Sci. 2001. 2:103–109.
Article16. Davis WC, Marusic S, Lewin HA, Splitter GA, Perryman LE, McGuire TC, Gorham JR. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet Immunol Immunopathol. 1987. 15:337–376.
Article17. Davis WC, Zuckermann FA, Hamilton MJ, Barbosa JIR, Saalmuller A, Binns RM, Licence ST. Analysis of monoclonal antibodies that recognize γδ T/null cells. Vet Immunol Immunopathol. 1998. 60:305–316.
Article18. De Smet W, Vaeck M, Smet E, Brys L, Hamers R. Rabbit leukocyte surface antigens defined by monoclonal antibodies. Eur J Immunol. 1983. 13:919–928.
Article19. Goddeeris BM. Pastoret PP, Griebel P, Bazin H, Govaerts A, editors. Immunology of cattle. Handbook of Vertebrate Immunology. 1998. San Diego: Academic Press;439–484.
Article20. Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993. 11:637–685.
Article21. Hamers R, Muyldermans S. Pastoret PP, Griebel P, Bazin H, Govaerts A, editors. Immunology of camels and llamas. Handbook of Vertebrate Immunology. 1998. San Diego: Academic Press;421–438.
Article22. Hamilton MJ, Davis WC. Davis WC, editor. Culture conditions that optimize outgrowth of hybridomas. Monoclonal Antibody Protocols. 1995. Totowa: Humana Press;17–28.
Article23. Haverson K, Saalmuller A, Alvarez B, Alonso F, Bailey M, Bianchi ATJ, Boersma WJA, Chen Z, Davis WC, Dominguez J, Engelhardt H, Ezquerra A, Grosmaire LS, Hamilton MJ, Hollemweguer E, Huang CA, Khanna KV, Kuebart G, Lackovic G, Ledbetter JA, Lee R, Llanes D, Lunney JK, McCullough KC, Molitor T, Nielsen J, Niewold TA, Pescovitz MD, Perez de la Lastra J, Rehakova Z, Salmon H, Schnitzlein WM, Seebach J, Simon A, Sinkora J, Sinkora M, Stokes CR, Summerfield A, Sver L, Thacker E, Valpotic I, Yang H, Zuckermann FA, Zwart R. Overview of the Third International Workshop on Swine Leukocyte Differentiation Antigens. Vet Immunol Immunopathol. 2001. 80:5–23.
Article24. Hein WR, Dudler L, Mackay CR. Surface expression of differentiation antigens on lymphocytes in the ileal and jejunal Peyer's patches of lambs. Immunology. 1989. 68:365–370.25. Hein WR, Mackay CR. Prominence of γδ T cells in the ruminant immune system. Immunol Today. 1991. 12:30–34.
Article26. Hopkins J, Ross A, Dutia BM. Summary of workshop findings of leukocyte antigens in sheep. Vet Immunol Immunopathol. 1993. 39:49–59.27. Howard CJ, Leibold W. Individual antigens of cattle. Bovine CD5 (BoCD5). Vet Immunol Immunopathol. 1991. 27:55–60.28. Howard CJ, Morrison WI. Comparison of reactivity of monoclonal antibodies on bovine, ovine and caprine tissues and on cells from other animal species. Vet Immunol Immunopathol. 1991. 27:32–34.
Article29. Howard CJ, Morrison WI, Bensaid A, Davis WC, Eskra L, Gerdes J, Hadam M, Hurley D, Leibold W, Letesson JJ, MacHugh N, Naessens J, O'Reilly K, Parsons KR, Schlote D, Sopp P, Splitter G, Wilson R. Summary of workshop findings for leukocyte antigens of cattle. Vet Immunol Immunopathol. 1991. 27:21–27.
Article30. Howard CJ, Naessens J. Summary of workshop findings for cattle (tables 1 and 2). Vet Immunol Immunopathol. 1993. 39:25–47.31. Kotani M, Yamamura Y, Tamatani T, Kitamura F, Miyasaka M. Generation and characterization of monoclonal antibodies against rabbit CD4, CD5 and CD11a antigens. J Immunol Methods. 1993. 157:241–252.
Article32. Kotani M, Yamamura Y, Tsudo M, Tamatani T, Kitamura F, Miyasaka M. Generation of monoclonal antibodies to the rabbit interleukin-2 receptor alpha chain (CD25) and its distribution in HTLV-1-transformed rabbit T cells. Jpn J Cancer Res. 1993. 84:770–775.
Article33. Kydd J, Antczak DF, Allen WR, Barbis D, Butcher G, Davis W, Duffus WPH, Edington N, Grunig G, Holmes MA, Lunn DP, McCulloch J, O'Brien A, Perryman LE, Tavernor A, Williamson S, Zhang C. Report of the First International Workshop on equine leucocyte antigens, Cambridge, UK, July 1991. Vet Immunol Immunopathol. 1994. 42:3–60.
Article34. Lanier LL, Allison JP, Phillips JH. Correlation of cell surface antigen expression on human thymocytes by multi-color flow cytometric analysis: implications for differentiation. J Immunol. 1986. 137:2501–2507.35. Lanier LL, Engleman EG, Gatenby P, Babcock GF, Warner NL, Herzenberg LA. Correlation of Functional Properties of Human Lymphoid Cell Subsets and Surface Marker Phenotypes Using Multiparameter Analysis and Flow Cytometry. Immunol Rev. 1983. 74:143–160.
Article36. Lunn DP, Holmes MA, Antczak DF, Agerwal N, Baker J, Bendali-Ahcene S, Blanchard-Channell M, Byrne KM, Cannizzo K, Davis W, Hamilton MJ, Hannant D, Kondo T, Kydd JH, Monier MC, Moore PF, O'Neil T, Schram BR, Sheoran A, Stott JL, Sugiura T, Vagnoni KE. Report of the second equine leucocyte antigen workshop, Squaw Valley, California, July 1995. Vet Immunol Immunopathol. 1998. 62:101–143.
Article37. Lunn DP, Holmes MA, Duffus WPH. Equine T-lymphocyte MHC II expression: variation with age and subset. Vet Immunol Immunopathol. 1993. 35:225–238.
Article38. Lunney JK, Walker K, Goldman T, Aasted B, Bianchi A, Binns R, Licence S, Bischof R, Brandon M, Blecha F, Kielian TL, McVey DS, Chu RM, Carr M, Howard C, Sopp P, Davis W, Dvorak P, Dominguez J, Canals A, Sanchez Vizcaino JM, Kim YB, Laude H, Mackay CR, Magnusson U, McCullough K, Misfeldt M, Murtaugh M, Molitor T, Choi C, Pabst R, Parkhouse RM, Denham S, Yang H, Pescovitz M, Pospisil R, Tlaskalova H, Saalmueller A, Weiland E, Salmon H, Sachs D, Arn S, Shimizu M, Stokes C, Stevens K, Valpotic I, Zuckermann F, Husmann R. Overview of the First International Workshop to define swine leukocyte cluster of differentiation (CD) antigens. Vet Immunol Immunopathol. 1994. 43:193–206.
Article39. Mackay CR, Hein WR. A large proportion of bovine T cells express the γδ T cell receptor and show a distinct tissue distribution and surface phenotype. Int Immunol. 1989. 1:540–545.
Article40. Mackay CR, Maddox JF, Wijffels GL, MacKay IR, Walker ID. Characterization of a 95,000 molecule on sheep leucocytes homologous to murine Pgp-1 and human CD44. Immunology. 1988. 65:93–99.41. Mage RG. Pastoret PP, Griebel P, Bazin H, Govaerts A, editors. Immunology of lagomorphs. Handbook of Vertebrate Immunology. 1998. San Diego: Academic Press;223–260.
Article42. Morrison WI, Davis WC. Individual antigens of cattle. Differentiation antigens expressed predominantly on CD4-CD8-T lymphocytes (WC1, WC2). Vet Immunol Immunopathol. 1991. 27:71–76.
Article43. Mossad AA, Elbagoury AR, Khalid AM, Waters WR, Tibary A, Hamilton MJ, Davis WC. Identification of monoclonal antibody reagents for use in the study of immune response in camel and water buffalo. Proc Int Sci Conf Camels. 2006. 13:2391–2411.44. Muriuki SP, Olaho-Mukani W, Naessens J. A panel of monoclonal antibodies that cross-react with leukocyte differentiation antigens from dromedary camel (Cameluus dromedarius). J Camel Prac Res. 1998. 5:179–185.45. Naessens J. Characterisation of lymphocyte populations in African buffalo (Syncerus caffer) and waterbuck (Kobus defassa) with workshop monoclonal antibodies. Vet Immunol Immunopathol. 1991. 27:153–162.
Article46. Naessens J, Hopkins J. Introduction and summary of workshop findings. Vet Immunol Immunopathol. 1996. 52:213–235.
Article47. Naessens J, Olubayo RO, Davis WC, Hopkins J. Cross-reactivity of workshop antibodies with cells from domestic and wild ruminants. Vet Immunol Immunopathol. 1993. 39:283–290.
Article48. Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984. 133:368–375.49. Pospisil R, Fitts MG, Mage RG. CD5 is a potential selecting ligand for B cell surface immunoglobulin framework region sequences. J Exp Med. 1996. 184:1279–1284.
Article50. Pospisil R, Obiakor H, Newman BA, Alexander C, Mage RG. Stable expression of the extracellular domains of rabbit recombinant CD5: development and characterization of polyclonal and monoclonal antibodies. Vet Immunol Immunopathol. 2005. 103:257–267.
Article51. Raman C, Knight KL. CD5+ B cells predominate in peripheral tissues of rabbit. J Immunol. 1992. 149:3858–3864.52. Saalmueller A, Denham S, Haverson K, Davis WC, Dominguez J, Pescovitz MD, Stokes CC, Zuckermann F, Lunney JK. The second International Swine CD Workshop. Vet Immunol Immunopathol. 1996. 54:155–158.
Article53. Saalmuller A, Aasted B, Canals A, Dominguez J, Goldman T, Lunney JK, Maurer S, Pescovitz MD, Pospisil R, Salmon H, Tlaskalova H, Valpotic I, Vizcaino JS, Weiland E, Zuckermann F. Summary of workshop findings for porcine T-lymphocyte antigens. Vet Immunol Immunopathol. 1994. 43:219–228.
Article54. Saalmuller A, Lunney JK, Daubenberger C, Davis W, Fischer U, Göbel TW, Griebel P, Hollemweguer E, Lasco T, Meister R, Schuberth HJ, Sestak K, Sopp P, Steinbach F, Xiao-Wei W, Aasted B. Summary of the animal homologue section of HLDA8. Cell Immunol. 2005. 236:51–58.
Article55. Saalmuller A, Pauly T, Lunney JK, Boyd P, Aasted B, Sachs DH, Arn S, Bianchi A, Binns RM, Licence S, Whyte A, Blecha F, Chen Z, Chu RM, Davis WC, Denham S, Yang H, Whittall T, Parkhouse RM, Dominguez J, Ezquerre A, Alonso F, Horstick G, Howard C, Sopp P, Kim YB, Lipp J, Mackay C, Magyar A, McCullough K, Arriens A, Summerfield A, Murtaugh M, Nielsen J, Novikov B, Pescovitz MD, Schuberth HJ, Leibold W, Schutt C, Shimizu M, Stokes C, Haverson K, Bailey M, Tlaskalova H, Trebichavsky I, Valpotic I, Walker J, Lee R, Zuckermann FA. Overview of the Second International Workshop to define swine cluster of differentiation (CD) antigens. Vet Immunol Immunopathol. 1998. 60:207–228.
Article56. Sopp P, Howard CJ. Cross-reactivity of monoclonal antibodies to defined human leucocyte differentiation antigens with bovine cells. Vet Immunol Immunopathol. 1997. 56:11–25.
Article57. Sopp P, Kwong LS, Howard CJ. Cross-reactivity with bovine cells of monoclonal antibodies submitted to the 6th International Workshop on Human Leukocyte Differentiation Antigens. Vet Immunol Immunopathol. 2001. 78:197–206.
Article58. Sopp P, Redknap L, Howard C. Cross-reactivity of human leucocyte differentiation antigen monoclonal antibodies on porcine cells. Vet Immunol Immunopathol. 1998. 60:403–408.
Article59. Tavernor AS, Deverson EV, Coadwell WJ, Lunn DP, Zhang C, Davis W, Butcher GW. Molecular cloning of equine CD44 cDNA by a COS cell expression system. Immunogenetics. 1993. 37:474–477.60. Tumas DB, Brassfield AL, Travenor AS, Hines MT, Davis WC, McGuire TC. Monoclonal antibodies to the equine CD2 T lymphocyte marker, to a pan-granulocyte/monocyte marker and to a unique pan-B lymphocyte marker. Immunobiology. 1994. 192:48–64.
Article61. Vilmos P, Kurucz E, Ocsovszki I, Keresztes G, Ando I. Phylogenetically conserved epitopes of leukocyte antigens. Vet Immunol Immunopathol. 1996. 52:415–426.
Article62. Wijngaard PLJ, MacHugh ND, Metzelaar MJ, Romberg S, Bensaid A, Pepin L, Davis WC, Clevers HC. Members of the novel WC1 gene family are differentially expressed on subsets of bovine CD4-CD8-γδ T-lymphocytes. J Immunol. 1994. 152:3476–3482.63. Wijngaard PLJ, Metzelaar MJ, MacHugh ND, Morrison WI, Clevers HC. Molecular characterization of the WC1 antigen expressed specifically on bovine CD4-CD8-γδ T lymphocytes. J Immunol. 1992. 149:3273–3277.64. Wilkinson JM, Galea-Lauri J, Sellars RA, Boniface C. Identification and tissue distribution of rabbit leucocyte antigens recognized by monoclonal antibodies. Immunology. 1992. 76:625–630.65. Wilkinson JM, Mcdonald G, Smith S, Galea-Lauri J, Lewthwaite J, Henderson B, Revell PA. Immunohistochemical identification of leucocyte populations in normal tissue and inflamed synovium of the rabbit. J Pathol. 1993. 170:315–320.
Article66. Zola H, Swart B, Nicholson I, Aasted B, Bensussan A, Boumsell L, Buckley C, Clark G, Drbal K, Engel P, Hart D, Horejsi V, Isacke C, Macardle P, Malavasi F, Mason D, Olive D, Saalmueller A, Schlossman SF, Schwartz-Albiez R, Simmons P, Tedder TF, Uguccioni M, Warren H. CD molecules 2005: human cell differentiation molecules. Blood. 2005. 106:3123–3126.
Article67. Zuckermann FA, Gaskins HR. Distribution of porcine CD4/CD8 double-positive T lymphocytes in mucosa-associated lymphoid tissues. Immunology. 1996. 87:493–499.
Article68. Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996. 87:500–512.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The use of crossreactive monoclonal antibodies to characterize the immune system of the water buffalo (Bubalus bubalis)
- Establishment of Reference Values for Platelet Activation Markers by Flow Cytometry
- Production of the Monoclonal Antibodies against Bartonella henselae Isolated from a Korean Patient
- From rabbit antibody repertoires to rabbit monoclonal antibodies
- The Structural Properties of Israeli Carp ( Cyprinus carpio L. ) Immunoglobulin