J Vet Sci.
2008 Mar;9(1):9-14. 10.4142/jvs.2008.9.1.9.
Decrease in intestinal endocrine cells in Balb/c mice with CT-26 carcinoma cells
- Affiliations
-
- 1Department of Radiological Science, College of Health Science, Catholic University of Daegu, Daegu 712-702, Korea.
- 2Department of Herbal Biotechnology, Daegu Haany University, Gyeongsan 712-715, Korea.
- 3Department of Anatomy and Histology, College of Oriental Medicine, Daegu Haany University, Gyeongsan 712-715, Korea. gucci200@hanmail.net
- 4Development Team for The New Drug of Oriental Medicine (BK21 Program), Daegu Haany University, Gyeongsan 712-715, Korea.
- KMID: 1102948
- DOI: http://doi.org/10.4142/jvs.2008.9.1.9
Abstract
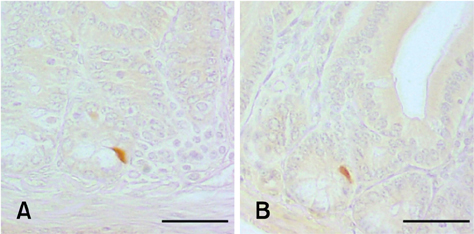
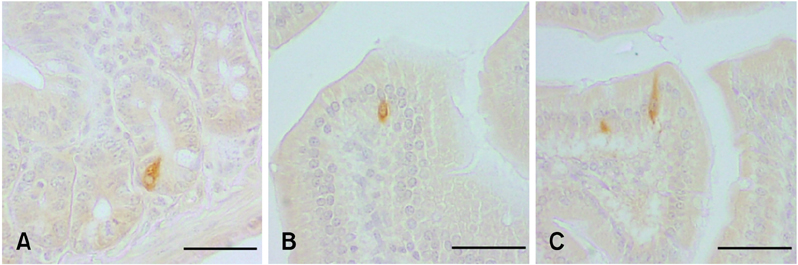
- The density of intestinal endocrine cells, in Balb/c mice with colon 26 (CT-26) carcinoma cells, were examined immunohistochemically at 28 days after implantation. After CT-26 cell administration there was a significant decrease in most of the intestinal endocrine cells (p < 0.01) compared with the control group. The significant quantitative changes in the intestinal endocrine cell density might contribute to the development of the gastrointestinal symptoms commonly encountered in cancer patients.
MeSH Terms
Figure
Reference
-
1. Buchan AM, Grant S, Brown JC, Freeman HJ. A quantitative study of enteric endocrine cells in celiac sprue. J Pediatr Gastroenterol Nutr. 1984. 3:665–671.
Article2. Cohn DV, Elting JJ, Frick M, Elde R. Selective localization of the parathyroid secretory protein-I/adrenal medulla chromogranin A protein family in a wide variety of endocrine cells of the rat. Endocrinology. 1984. 114:1963–1974.
Article3. D'Este L, Buffa R, Pelagi M, Siccardi AG, Renda T. Immunohistochemical localization of chromogranin A and B in the endocrine cells of the alimentary tract of the green frog, Rana esculenta. Cell Tissue Res. 1994. 277:341–349.4. DeWys WD. Studies correlating the growth rate of a tumor and its metastases and providing evidence for tumor-related systemic growth-retarding factors. Cancer Res. 1972. 32:374–379.5. El-Salhy M, Sitohy B. Abnormal gastrointestinal endocrine cells in patients with diabetes type I: relationship to gastric emptying and myoelectrical activity. Scand J Gastroenterol. 2001. 36:1162–1169.6. Guyton AC. Guyton AC, John EH, editors. Secretory functions of the alimentary tract. Textbook of Medical Physiology. 2006. 11th ed. Philadelphia: Saunders;791–807.7. Ham TS, Lee HS, Lee JH, Ku SK. Changes of gastrointestinal chromogranin-immunoreactive cells after implantation of Colon-26 murine carcinoma cells in Balb/c mouse. Lab Anim Res. 2005. 21:179–184.8. Kitamura N, Yamada J, Calingasan NY, Yamashita T. Immunocytochemical distribution of endocrine cells in the gastro-intestinal tract of the horse. Equine Vet J. 1984. 16:103–107.
Article9. Komurcu S, Nelson KA, Walsh D, Ford RB, Rybicki LA. Gastrointestinal symptoms among inpatients with advanced cancer. Am J Hosp Palliat Care. 2002. 19:351–355.
Article10. Ku SK, Lee HS, Lee JH. An immunohistochemical study of gastrointestinal endocrine cells in the Balb/c mouse. Anat Histol Embryol. 2004. 33:42–48.
Article11. Ku SK, Lee HS, Lee JH. An immunohistochemical study of the gastrointestinal endocrine cells in the C57BL/6 mice. Anat Histol Embryol. 2003. 32:21–28.
Article12. Ku SK, Seong SK, Kim DY, Lee HS, Kim JD, Choi HY, Seo BI, Lee JH. Changes of the intestinal endocrine cells in the C57BL/6 mouse after implantation of murine lung carcinoma (3LL): an immunohistochemical quantitative study. World J Gastroenterol. 2005. 11:5460–5467.
Article13. Ravazzola M, Baetens D, Engerman R, Kovacevic N, Vranic M, Orci L. Endocrine cells in oxyntic mucosa of a dog 5 years after pancreatectomy. Horm Metab Res. 1977. 9:480–483.
Article14. Rode J, Dhillon AP, Papadaki L, Stockbrügger R, Thompson RJ, Moss E, Cotton PB. Pernicious anaemia and mucosal endocrine cell proliferation of the non-antral stomach. Gut. 1986. 27:789–798.
Article15. Shimamoto F, Tahara E, Yanaihara N. Gut endocrine cells in rat intestinal-tract carcinoma induced by 1, 2-dimethylhydrazine. J Cancer Res Clin Oncol. 1983. 105:221–230.
Article16. Solcia E, Usellini L, Buffa R, Rindi G, Villani L, Aguzzi A, Silini E. Polak JM, editor. Endocrine cells producing regulatory peptides. Regulatory Peptides. 1989. Basel: Birkhauser;220–246.
Article17. Sternberger LA. Sternberger LA, editor. The unlabeled antibody peroxidase-antiperoxidase (PAP) method. Immunocytochemistry. 1979. New York: John Wiley & Sons;104–169.18. Yasui W, Sumiyoshi H, Hata J, Mandai K, Tahara E. Gut endocrine cells in rat stomach carcinoma induced by N-methyl-N'-nitro-N-nitrosoguanidine. J Cancer Res Clin Oncol. 1986. 111:87–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Studies on the Serotonin, Somatostatin and Gastrin-Positive Cells in the Gastric Adenocarcinoma
- Immunohistochemical Study of the Pancreatic Endocrine Cells in the BALB/c mice: An Unique Distributional Pattern of Glucagon
- Regional Distribution and Relative Frequency of Gastrointestinal Endocrine Cells in Large Intestines of C57BL/6 Mice
- Changes of gastrointestinal argyrophil endocrine cells in the COLO205 tumor-implanted Balb/c-nu/nu mice
- Intestinal mastocytosis and goblet cell hyperplasia in BALB/c and C3H mice infected with Neodiplostomum seoulense