Korean J Ophthalmol.
2008 Jun;22(2):92-99. 10.3341/kjo.2008.22.2.92.
The Therapeutic Effects of Bevacizumab in Patients with Polypoidal Choroidal Vasculopathy
- Affiliations
-
- 1Department of Ophthalmology, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea. yhyoon@amc.seoul.kr
- KMID: 1099002
- DOI: http://doi.org/10.3341/kjo.2008.22.2.92
Abstract
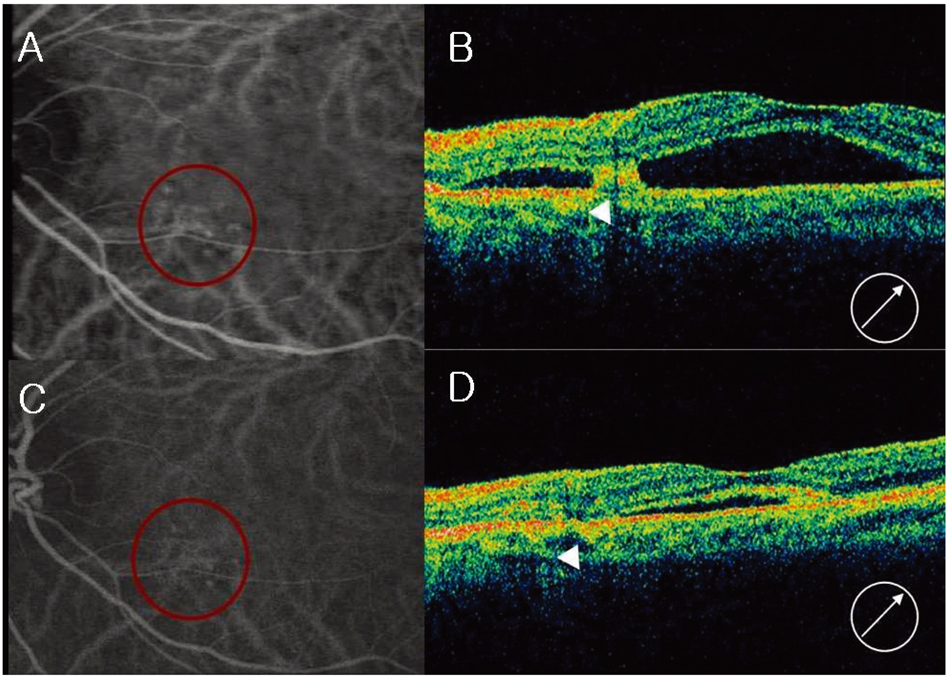
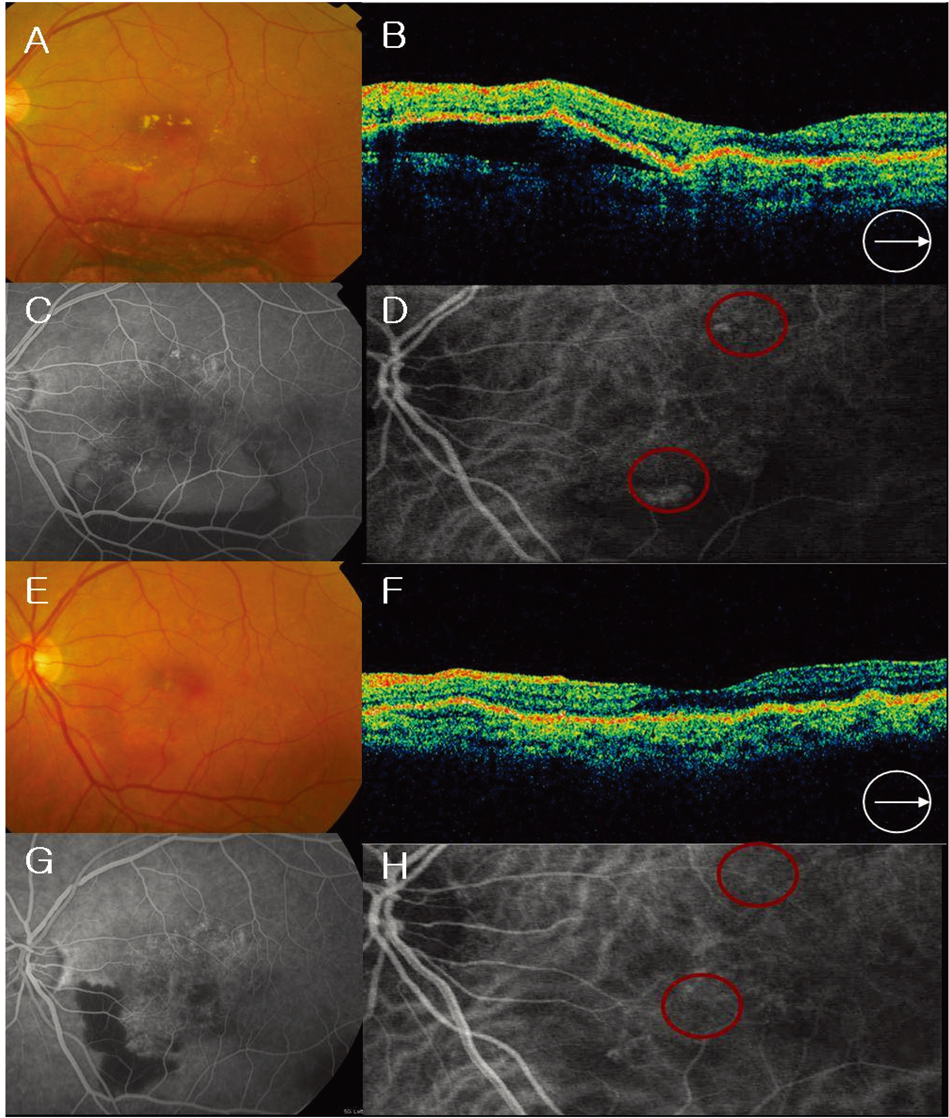
- PURPOSE: To evaluate the efficacy and safety of intravitreal bevacizumab for polypoidal choroidal vasculopathy (PCV). METHODS: In this retrospective interventional pilot study, 12 eyes of 11 patients with active PCV were treated with intravitreal bevacizumab (1.25 mg) alone or in combination with photodynamic therapy (PDT) depending on the informed patient's choice. Intravitreal bevacizumab was repeated at 6-week intervals until the regression of active lesion was detected on fluorescein angiography (FA) which was done on a regular basis, Indocyanine green angiography (ICGA) and optical coherence tomography (OCT) analyses. RESULTS: Intravitreal bevacizumab was given alone in 8 eyes (Group 1) and in combination with PDT in 4 eyes (Group 2). Mean follow-up duration was 17 weeks in group 1 and 15 weeks in group 2 after bevacizumab treatment. The mean number of bevacizumab injections was 2.2 in group 1 and 2.5 in group 2. Mean BCVA improved from 20/63 to 20/40 in group 1 and 20/63 to 20/32 in group 2. Of all eyes, the BCVA improved by > or =2 lines in seven (58%) eyes and resolution of fluid and hemorrhages in clinical examination, an absence of leakage on repeat FAs, or resolved pigment epithelial detachment (PED) and/or subretinal fluid (SRF) on OCT exam was confirmed in 10 (83%) eyes. Partial or complete regression of the polypoidal vessels and interconnecting vessels was reported for most cases at the last follow-up. No significant ocular or systemic side effects were observed in both groups. CONCLUSIONS: Short-term results indicate that intravitreal bevacizumab (1.25 mg) alone or in combination with PDT is well tolerated and associated with improvement in BCVA and reduced angiographic leakage in most patients. Further evaluation of intravitreal bevacizumab therapy for the treatment of PCV is warranted.
MeSH Terms
-
Aged
Aged, 80 and over
Angiogenesis Inhibitors/adverse effects/*therapeutic use
Antibodies, Monoclonal/adverse effects/*therapeutic use
Choroid/*blood supply/pathology
Coloring Agents/diagnostic use
Combined Modality Therapy
Female
Fluorescein Angiography
Humans
Indocyanine Green/diagnostic use
Injections
Male
Middle Aged
Peripheral Vascular Diseases/diagnosis/*drug therapy/physiopathology
*Photochemotherapy
Pilot Projects
Retrospective Studies
Tomography, Optical Coherence
Treatment Outcome
Vascular Endothelial Growth Factor A/antagonists & inhibitors
Visual Acuity/physiology
Vitreous Body
Figure
Cited by 1 articles
-
Combined Photodynamic Therapy and Intravitreal Bevacizumab Injection for Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy
Mi Hyun Lee, Gi Hong Gu, Ji Eun Lee, Boo Sub Oum
J Korean Ophthalmol Soc. 2011;52(7):816-824. doi: 10.3341/jkos.2011.52.7.816.
Reference
-
1. Yannuzzi LA, Sorenson J, Spaide RF, et al. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990. 10:1–8.2. Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995. 15:100–110.3. Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol. 1999. 117:1503–1510.4. Uyama M, Matsubara T, Fukushima I, et al. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999. 117:1035–1042.5. Moorthy RS, Lyon AT, Rabb MF, et al. Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology. 1998. 105:1380–1385.6. Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthal. 1997. 115:478–485.7. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002. 133:639–648.8. Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003. 47:379–384.9. Hattenbach LO, Klais C, Koch FH, Gümbel HO. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001. 108:1485–1492.10. Shirage F, Matsuo T, Yokoe S, et al. Surgical treatment of submacular hemorrhage associated with idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 1999. 128:147–154.11. Terasaki H, Miyake Y, Suzuki T, et al. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002. 86:321–327.12. Spaide RF, Donsoff I, Lam DL, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002. 22:529–535.13. Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004. 111:1576–1584.14. Ojima Y, Tsujikawa A, Otani A, et al. Recurrent bleeding after photodynamic therapy in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2006. 141:958–960.15. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005. 36:331–335.16. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006. 113:363–372.17. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006. 26:383–390.18. Nguyen QD, Shah S, Tatlipinar S, et al. Bevacizumab suppresses choroidal neovascularizsation caused by pathological myopia. Br J Ophthalmol. 2005. 89:1368–1370.19. Tong JP, Chan WM, Liu DT, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006. 141:456–462.20. Matsuoka M, Ogata N, Otsuji T, et al. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004. 88:809–815.21. Lafaut BA, Aisenbrey S, Van den Broecke C, et al. Polypoidal choroidal vasculopathy pattern in age-related macular degeneration: a clinicopathologic correlation. Retina. 2000. 20:650–654.22. VEGF inhibition study in ocular neovascularization clinical trial group, Chakravarthy U, Adamis AP, Cunningham ET Jr, et al. Year 2 efficacy results of 2 randomized controlled clinical trial of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006. 113:1508–1525.23. Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006. 113:633.e1–633.e4.24. Mauget-Faysse M, Quaranta-El Maftouhi M, De La Marnierre E, Leys A. Photodynamic therapy with verteporfin in the treatment of exudative idiopathic polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2006. 16:695–704.25. Lafaut BA, Leys AM, Snyers B, et al. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000. 238:752–759.26. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004. 49:25–37.27. Tsujikawa A, Sasahara M, Otani A, et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007. 143:102–111.28. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003. 121:1392–1396.29. Silva RM, Figueira J, Cachulo ML, et al. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2005. 243:973–979.30. Lee SC, Seong YS, Koh HJ, et al. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004. 218:193–201.31. Chan WM, Liu DT, Lai TY, et al. Extensive submacular haemorrhage in polypoidal choroidal vasculopathy managed by sequential gas displacement and photodynamic therapy: a pilot study of one-year follow up. Clin Experiment Ophthalmol. 2005. 33:611–618.32. Augustin AJ, Schmidt-Erfurth U. Verteporfin therapy combined with intravitreal triamcinolone in all types of choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006. 113:14–22.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Bevacizumab With or Without Photodynamic Therapy for the Treatment of Polypoidal Choroidal Vasculopathy
- Photodynamic Therapy with Verteporfin in Polypoidal Choroidal Vasculopathy
- Short-term Efficacy of Intravitreal Bavacizumab for Polypoidal Choroidal Vasculopathy
- Combined Photodynamic Therapy and Intravitreal Bevacizumab Injection for Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy
- A Case of Subretinal Hematoma Secondary to Polypoidal Choroidal Vasculopathy Misunderstood as a Subretinal Mass