Korean J Lab Med.
2009 Jun;29(3):256-261. 10.3343/kjlm.2009.29.3.256.
A Case of Lambda-expressing Pulmonary MALT Lymphoma with Dual Clonal Rearrangements of Kappa and Lambda Immunoglobulin Light Chain Gene
- Affiliations
-
- 1Department of Laboratory Medicine, Chosun University Medical School, Gwangju, Korea. sjbjang@chosun.ac.kr
- 2Department of Pathology, Chosun University Medical School, Gwangju, Korea.
- 3Research Center for Resistant Cells, Chosun University Medical School, Gwangju, Korea.
- KMID: 1096979
- DOI: http://doi.org/10.3343/kjlm.2009.29.3.256
Abstract
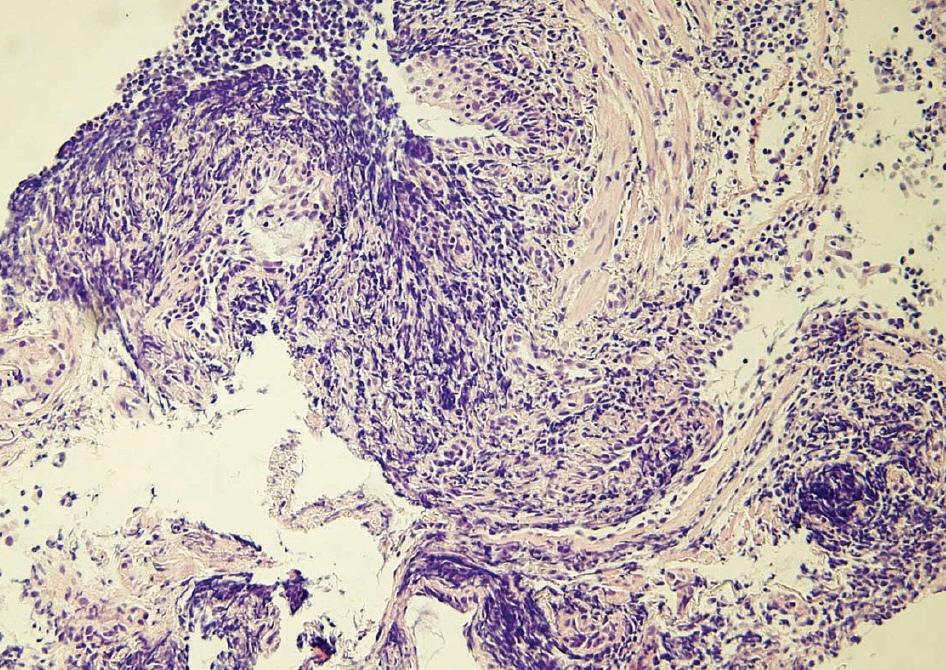
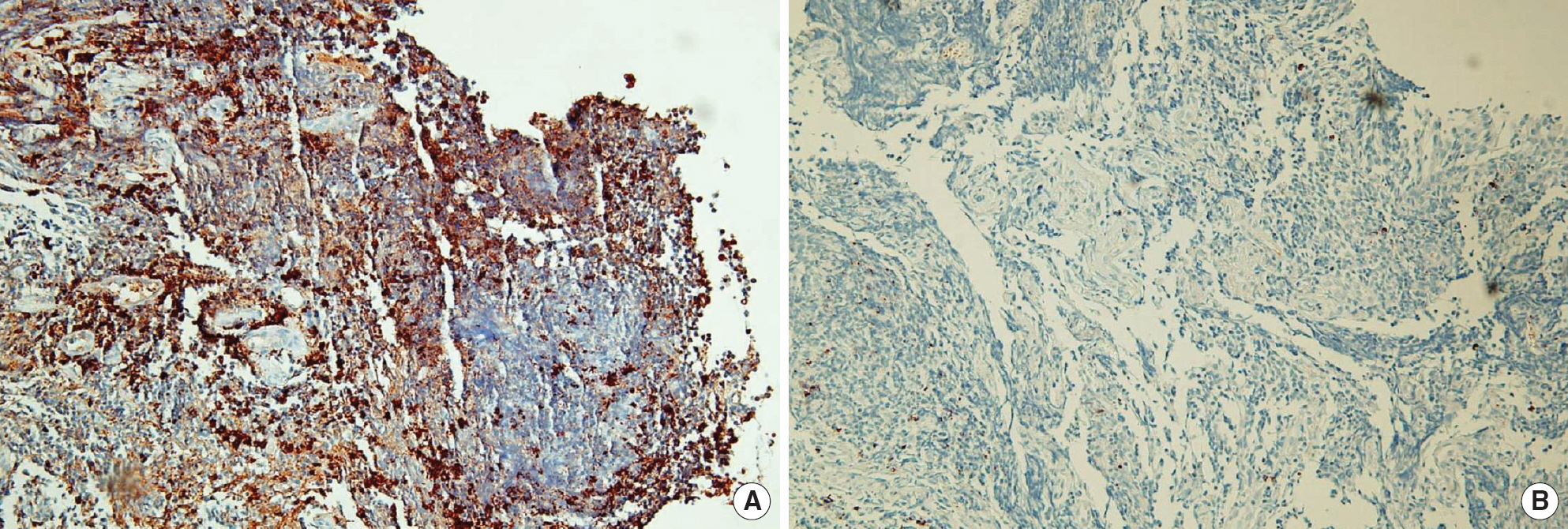
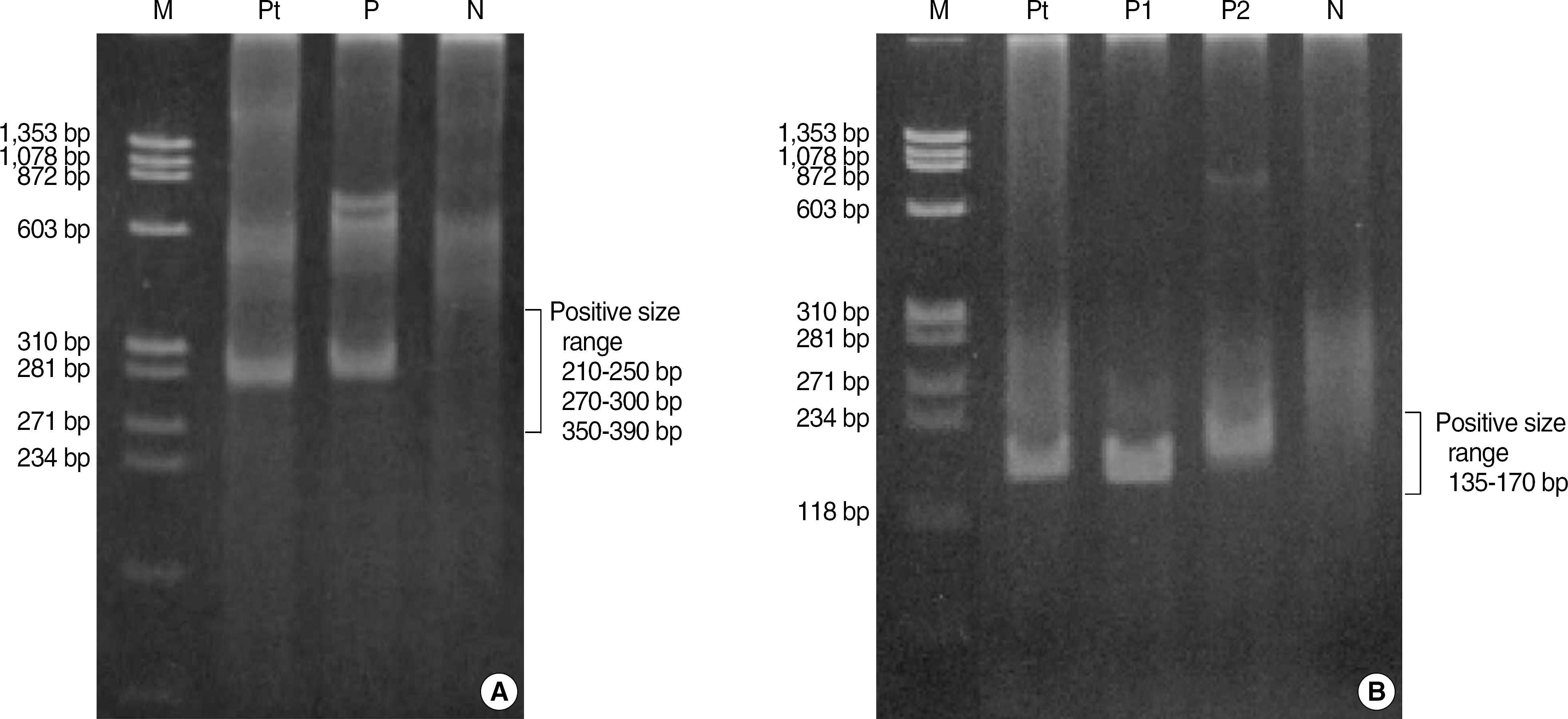
- A 70-yr-old woman was hospitalized with a history of dry cough. Bronchial endoscopy and transbronchial lung biopsy were performed. However, the findings of histopathology and immunohistochemistry were not sufficient to decide whether the lesion was benign or malignant, because of the presence of crush artifacts in the biopsy specimens. We performed B-cell clonality studies using BIOMED-2 multiplex PCR (InVivoScribe Technologies, USA) to detect clonal rearrangements in the immunoglobulin gene. The results of multiplex PCR showed clonal rearrangements of both kappa and lambda immunoglobulin light chain genes. The findings of immunochemistry revealed that the lesion expressed lambda light chain, but not kappa light chain. Based on the clinical, pathologic, and molecular findings, this case was diagnosed as pulmonary MALT lymphoma. We report the first case in Korea of lambda-expressing MALT lymphoma that is shown to have dual clonal rearrangements of kappa and lambda immunoglobulin light chain gene by multiplex PCR.
MeSH Terms
Figure
Cited by 2 articles
-
Analysis of Immunoglobulin Gene Rearrangement: Comparison between BIOMED-2 Multiplex PCR and Conventional Nested PCR
Ji-Youn Sung, So Young Kang, Sun-Hee Kim, Ji Eun Kwon, Young-Hyeh Ko
Lab Med Online. 2011;1(4):195-201. doi: 10.3343/lmo.2011.1.4.5.Clinical Significance of Clonal Rearrangement of the Immunoglobulin Gene in the Bone Marrow of Patients with B-cell Non-Hodgkin Lymphoma
Ji Hyun Kim, Ja Young Lee, Jong Ae Son, Sae Am Song, Seung Hwan Oh, Jeong Hwan Shin, Hye Ran Kim, Kyung Ran Jun, Jeong Nyeo Lee
Lab Med Online. 2014;4(3):125-131. doi: 10.3343/lmo.2014.4.3.125.
Reference
-
1.Langerak AW., Molina TJ., Lavender FL., Pearson D., Flohr T., Sambade C, et al. Polymerase chain reaction-based clonality testing in tissue samples with reactive lymphoproliferations: usefulness and pitfalls. A report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2007. 21:222–9.
Article2.Arnold A., Cossman J., Bakhshi A., Jaffe ES., Waldmann TA., Korsmeyer SJ. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983. 309:1593–9.
Article3.van Dongen JJ., Wolvers-Tettero IL. Analysis of immunoglobulin and T cell receptor genes. Part II: Possibilities and limitations in the diagnosis and management of lymphoproliferative diseases and related disorders. Clin Chim Acta. 1991. 198:93–174.4.Bourguin A., Tung R., Galili N., Sklar J. Rapid, nonradioactive detection of clonal T-cell receptor gene rearrangements in lymphoid neoplasms. Proc Natl Acad Sci U S A. 1990. 87:8536–40.
Article5.van Dongen JJ., Langerak AW., Bruggemann M., Evans PA., Hummel M., Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003. 17:2257–317.
Article6.van Krieken JH., Langerak AW., Macintyre EA., Kneba M., Hodges E., Sanz RG, et al. Improved reliability of lymphoma diagnostics via PCR-based clonality testing: report of the BIOMED-2 Concerted Action BHM4-CT98-3936. Leukemia. 2007. 21:201–6.7.Sandberg Y., van Gastel-Mol EJ., Verhaaf B., Lam KH., van Dongen JJ., Langerak AW. BIOMED-2 multiplex immunoglobulin/T-cell receptor polymerase chain reaction protocols can reliably replace Southern blot analysis in routine clonality diagnostics. J Mol Diagn. 2005. 7:495–503.
Article8.Isaacson PG., Muller-Hermelink HK., Piris MA., Berger F., Nathwani BN., Swerdlow SH, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). Jaffe ES, Harris NL, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press;2001. p. 157–60.9.Schmid C., Isaacson PG. Proliferation centres in B-cell malignant lymphoma, lymphocytic (B-CLL): an immunophenotypic study. Histopathology. 1994. 24:445–51.
Article10.Jeong SY., Chang YH., Lee JK., Hong YJ., Hong SI., Lee SS. Incidence and histologic patterns of bone marrow involvement of malignant lymphoma based on the World Health Organization classification-a single institution study. Korean J Lab Med. 2007. 27:383–7. (정소연,장윤환, 이진경, 홍영준, 홍석일, 이승숙. 세계보건기구 분류에 근거한 악성림프종의 골수침습 빈도 및 양상 - 단일 기관 연구. 대한진단검사의학회지 2007;27:383-7.).11.Saltzstein SL. Extranodal malignant lymphomas and pseudolymphomas. Pathol Annu. 1969. 4:159–84.12.Burke JS. Extranodal lymphoid proliferations: general principles and differential diagnosis. Knowles DM, editor. Neoplastic hematopathology. 2nd ed.New York: Lippincott, Williams & Wilkins;2001. p. 1165–82.13.Streubel B., Simonitsch-Klupp I., Mullauer L., Lamprecht A., Huber D., Siebert R, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004. 18:1722–6.
Article14.Rassenti LZ., Kipps TJ. Lack of allelic exclusion in B cell chronic lymphocytic leukemia. J Exp Med. 1997. 185:1435–45.
Article15.Greiner T., Armitage JO., Gross TG. Atypical lymphoproliferative diseases. Hematology Am Soc Hematol Educ Program. 2000. 133–46.
Article16.Zhang Y., Schlegelberger B., Plendl H., Sonnen R., Kuse R., Feller AC, et al. Clonal t(8;14)(p11;q31) in a case of reactive lymphoproliferation. Genes Chromosomes Cancer. 1993. 7:165–8.
Article17.Morales AV., Arber DA., Seo K., Kohler S., Kim YH., Sundram UN. Evaluation of B-cell clonality using the BIOMED-2 PCR method effectively distinguishes cutaneous B-cell lymphoma from benign lymphoid infiltrates. Am J Dermatopathol. 2008. 30:425–30.
Article18.Kubagawa H., Cooper MD., Carroll AJ., Burrows PD. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci USA. 1989. 86:2356–60.
Article19.Giachino C., Padovan E., Lanzavecchia A. kappa+lambda+ dual receptor B cells are present in the human peripheral repertoire. J Exp Med. 1995. 181:1245–50.20.Fujiwara T., Ishizawa K., Kohata K., Yamamoto J., Yamada MF., Kameoka J, et al. Aggressive B-cell lymphoma with dual surface immunoglobulin light-chain expression. Intern Med. 2007. 46:1458–61.
Article21.Gerdes T., Wabl M. Autoreactivity and allelic inclusion in a B cell nuclear transfer mouse. Nat Immunol. 2004. 5:1282–7.
Article22.Peltomaki P., Bianchi NO., Knuutila S., Teerenhovi L., Elonen E., Leskinen R, et al. Immunoglobulin kappa and lambda light chain dual genotype rearrangement in a patient with kappa-secreting B-CLL. Eur J Cancer Clin Oncol. 1988. 24:1233–8.23.Kaleem Z., Zehnbauer BA., White G., Zutter MM. Lack of expression of surface immunoglobulin light chains in B-cell non-Hodgkin lymphomas. Am J Clin Pathol. 2000. 113:399–405.
Article24.Nakagawa M., Seto M., Hosokawa Y. Molecular pathogenesis of MALT lymphoma: two signaling pathways underlying the antiapoptotic effect of API2-MALT1 fusion protein. Leukemia. 2006. 20:929–36.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Immunoglobulin lambda Light Chain Repertoire in Systemic Lupus Erythematosus
- Clinical Significance of Clonal Rearrangement of the Immunoglobulin Gene in the Bone Marrow of Patients with B-cell Non-Hodgkin Lymphoma
- Discrimination of Monoclonal Gammopathy Using Immunoassay for Free Light Chains
- Plasma Cell Cheilitis: A Clinicopathological and Immunohistochemical Study of 13 Cases
- Non-Radioactive Detection of Clonality in Malignant Lymphoid Neoplasms using the Polymerase Chain Reaction