J Korean Med Sci.
2011 Jul;26(7):893-899. 10.3346/jkms.2011.26.7.893.
Antiproliferation and Redifferentiation in Thyroid Cancer Cell Lines by Polyphenol Phytochemicals
- Affiliations
-
- 1Department of Surgery, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 2Department of Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Breast and Endocrine Surgery, Hallym Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. lskim0503@hallym.or.kr
- KMID: 1094260
- DOI: http://doi.org/10.3346/jkms.2011.26.7.893
Abstract
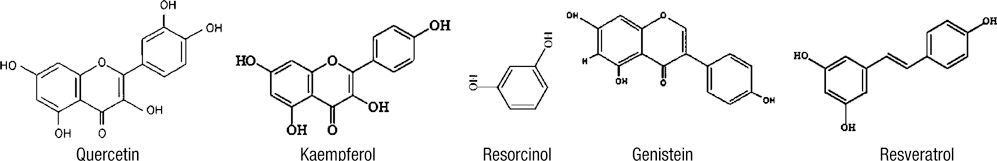
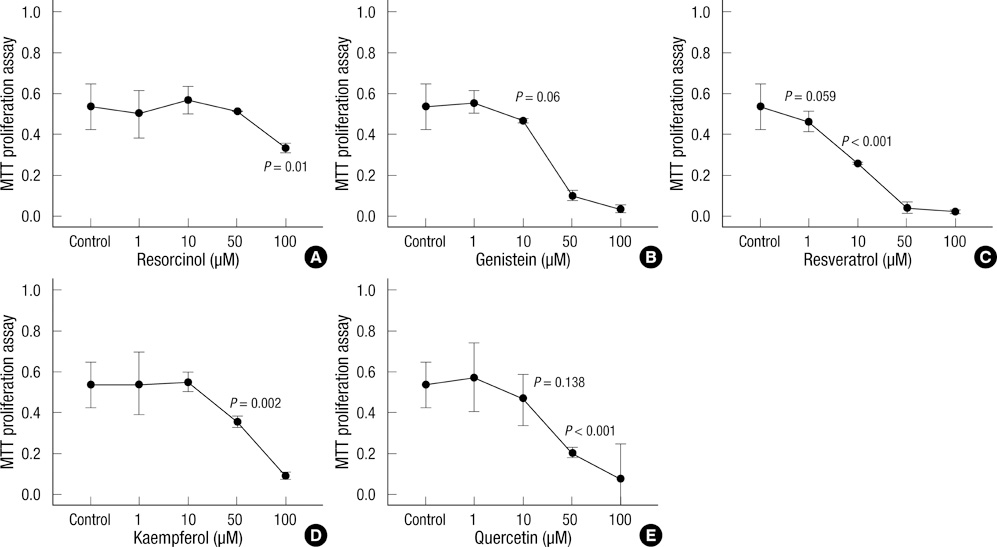
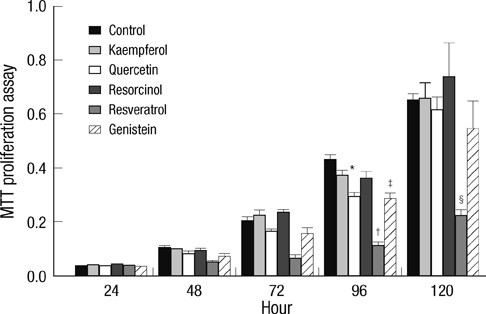
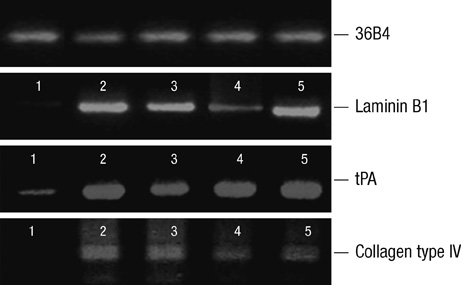
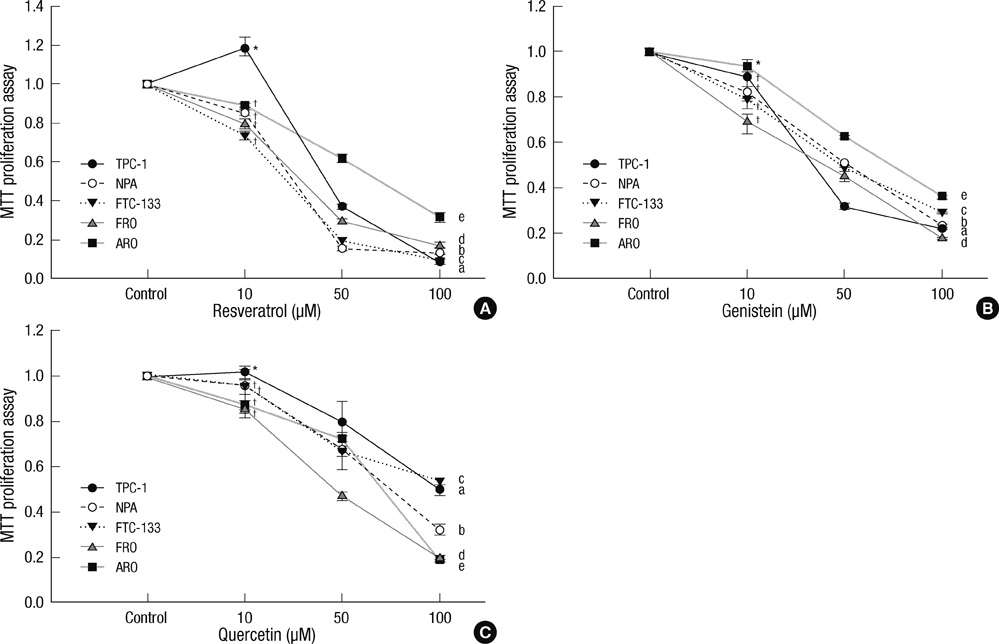
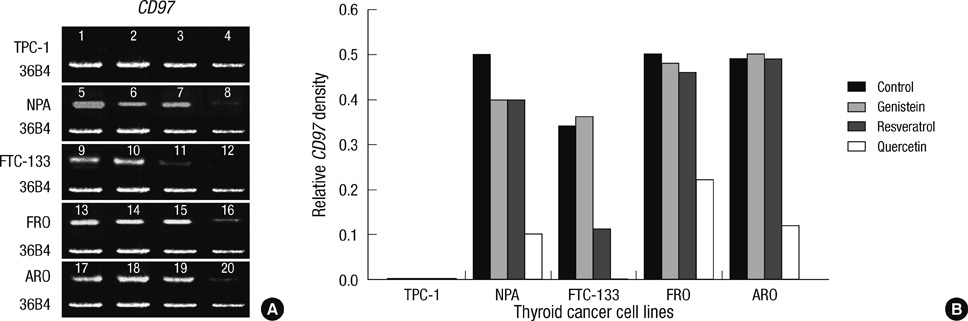
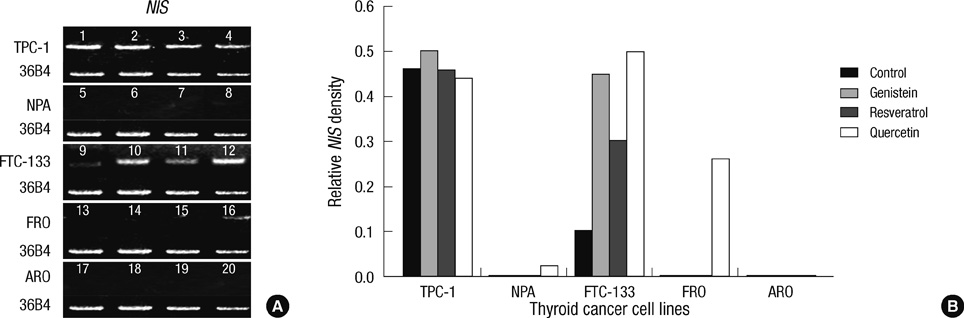
- Thyroid carcinogenesis is accompanied by loss of thyroid-specific functions and refractory to radioiodine and thyroid stimulating hormone (TSH) suppression therapy. Redifferentiating agents have been shown to inhibit tumor growth and improve the response to conventional therapy. Polyphenol phytochemicals (PPs) in fruits and vegetables have been reported to inhibit cancer initiation, promotion, progression and induce redifferentiation in selected types. In this study we examined PPs induce redifferentiation in thyroid cancer cell lines. We investigated the effects of genistein, resveratrol, quercetin, kaempferol, and resorcinol on the F9 embryonal carcinoma cell differentiation model. The thyroid cancer cell lines, TPC-1, FTC-133, NPA, FRO, and ARO, displayed growth inhibition in response to genistein, resveratrol, quercetin. We further demonstrated that genistein decreased the dedifferention marker CD97 in NPA cells and resveratrol decreased CD97 in FTC-133, NPA, FRO cells and quercetin decreased CD97 in all cell lines. We observed increased expression of differentiation marker NIS in FTC-133 cells in response to genistein, and resveratrol but no change in NPA, FRO, ARO cells. Quercetin increased or induced NIS in FTC-133, NPA, FRO cells. These findings suggest that PPs may provide a useful therapeutic intervention in thyroid cancer redifferentiation therapy.
Keyword
MeSH Terms
-
Antigens, CD/metabolism
Antineoplastic Agents/*pharmacology/therapeutic use
Carcinoma, Embryonal/*drug therapy/metabolism
Cell Differentiation/*drug effects
Cell Line, Tumor
Cell Proliferation/*drug effects
Flavonoids/*pharmacology/therapeutic use
Gene Expression Regulation, Neoplastic
Genistein/pharmacology/therapeutic use
Humans
Kaempferols/pharmacology/therapeutic use
Models, Biological
Phenols/*pharmacology/therapeutic use
Quercetin/pharmacology/therapeutic use
Resorcinols/pharmacology/therapeutic use
Stilbenes/pharmacology/therapeutic use
Symporters/metabolism
Thyroid Neoplasms/*drug therapy/metabolism
Figure
Reference
-
1. Park JW, Clark OH. Redifferentiation therapy for thyroid cancer. Surg Clin North Am. 2004. 84:921–943.2. Schmutzler C, Koehrle J. Innovative strategies for treatment of thyroid cancer. Eur J Endocrinol. 2000. 143:15–24.3. Lazar V, Bidart JM, Caillou B, Mahé C, Lacroix L, Fletti S, Schlumberger M. Expression of the Na+/I- symporter gene in human thyroid tumors: a comparison study with other thyroid specific genes. J Clin Endocrinol Metab. 1999. 84:3228–3234.4. Hoang-Vu C, Bull K, Schwarz I, Krause G, Schmutzler C, Aust G, Köhrle J, Dralle H. Regulation of CD97 protein in thyroid carcinoma. J Clin Endocrinol Metab. 1999. 84:1104–1109.5. Shen WI, Chung WY. Treatment of thyroid cancer with histone deacetylase inhibitor and peroxisome proliferater-activated receptor-γ agonists. Thyroid. 2005. 15:594–599.6. Zarnegar R, Brunaud L, Kanauchi H, Wong M, Fung M, Ginzinger D, Duh QA, Clark OH. Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery. 2002. 132:984–990.7. Park JC, Ryu DH, Park JW, Clark OH. Redifferentiation effects of troglitazone in human thyroid cancer cell lines. J Korean Surg Soc. 2004. 67:93–99.8. Chen YC, Shen SC, Chow JM, Ko CH, Tseng SW. Flavone inhibition of tumor growth via apoptosis in vitro and in vivo. Int J Oncol. 2004. 25:661–670.9. Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee TS, Lee SJ. Induction of cell cycle arrest and apotosis in human breast cancer cells by quercetin. Int J Oncol. 2001. 19:837–844.10. Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005. 81:1 Suppl. 284S–291S.11. Yin F, Giuliano AE, Van Herle AJ. Growth inhibitory effects of flavonoids in human thyroid cancer cell line. Thyroid. 1999. 9:369–376.12. Asou H, Koshizuka K, Kyo T, Takata N, Kamada N, Koeffier HP. Resveratrol, a natural product derived from grapes, is a new inducer of differentiation in human myeloid leukemias. Int J Hematol. 2002. 75:528–533.13. Dihal AA, Woutersen RA, van Ommen B, Rietjens IM, Stierum RH. Modulatory effects of quercetin on proliferation and differentiation of the human colorectal cell line Caco-2. Cancer Lett. 2006. 238:248–259.14. Nakamura Y, Chang CC, Mori T, Sato K, Ohtsuki K, Upham BL, Trosko JE. Augmentation of diffentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis. 2005. 26:665–671.15. Alonso A, Breuer B, Steuer B, Fisher J. The F9-EC line as a model for the analysis of differentiation. Int J Dev Biol. 1991. 35:389–397.16. Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006. 71:1397–1421.17. Gulati N, Laudet B, Zohravian VM, Murali R, Jhanwa-Uniyal M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006. 26:1177–1181.18. Mertens-Talcott SU, Bomser JA, Romero C, Talcott ST, Percival SS. Ellagic acid potentiates the effect of quercerin on p21waf1/cip1, p53, and MAP-kinase without affecting intracellular generation of reactive oxygen species in vitro. J Nutr. 2005. 135:609–614.19. Ramos S. Effect of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007. 18:427–442.20. Folman Y, Pope GS. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other utero-vaginotrophic compounds of low potency. J Endocrinol. 1966. 34:215–225.21. Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alph- or beta mediated response in transfected breast cancer cells. Exp Biol Med (Maywood). 2005. 230:558–568.22. Venkataraman GM, Yatin M, Marcinek R, Ain KB. Restoration of iodine uptake in dedifferentiated thyroid carcinoma: relatioship to human Na+/I- symporter gene methylation status. J Clin Endocrinol Metab. 1999. 84:2449–2457.23. Das PM, Singal R. DNA methyation and cancer. J Clin Oncol. 2004. 22:4632–4642.24. Lee WJ, Shim JY, Zhu BT. Mechanism for the inhibiton of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005. 68:1018–1030.25. Fang MZ, Wang Y, Ai N, Hou AZ, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003. 63:7563–7570.26. Ulrich S, Loitsch SM, Rau O, von Knethen A, Brüne B, Schubert-Zsilavecz M, Stein JM. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006. 66:7348–7354.27. Schmutzler C, Köehrle J. Retinoic acid redifferentiation therapy for thyroid cancer. Thyroid. 2000. 10:393–406.28. Elisei R, Vivaldi A, Agate L, Ciampi R, Monlinaro E, Piampiani P, Romei C, Favaiana P, Basolo F, Miccoli P, Capodano A, Collecchi P, Pacini F, Pinchera A. All-trans-Retinoic acid treatment inhibits the growth of retinoic acid receptor β messenger ribonucleic acid expressing thyroid cancer cell lines but does not reinduce the expression of thyroid-specific genes. J Clin Endocrinol Metab. 2005. 90:2403–2411.29. Hoque MO, Rosenbaum E, Westra WH, Xing M, Ladenson P, Zeiger MA, Sidransky D, Umbricht CB. Quantitative assessment of promoter methylation profiles in thyroid neoplasm. J Clin Endocrinol Metab. 2005. 90:4011–4018.30. Gardner CD, Jenny B, Liu JP, Feldman D, Franke AA, Brooks JD. Prostate soy isoflavone concentrations exceed serum levels after dietary supplementation. Prostate. 2009. 69:719–726.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter to the Editor: Antiproliferation and Redifferentiation in Thyroid Cancer Cell Line by Polyphenol Phytochemicals
- The Author Response: Antiproliferation and Redifferentiation in Thyroid Cancer Cell Line by Polyphenol Phytochemicals
- Redifferentiation Effects of Troglitazone in Human Thyroid Cancer Cell Lines
- Preclinical Models of Follicular Cell-Derived Thyroid Cancer: An Overview from Cancer Cell Lines to Mouse Models
- Sonic Hedgehog Protein Expression in Various Thyroid Tissues and Its Clinical Implication