J Vet Sci.
2007 Sep;8(3):219-222. 10.4142/jvs.2007.8.3.219.
Sertoli cell proliferation during the post hatching period in domestic fowl
- Affiliations
-
- 1Department of Histology and Embryology, Faculty of Veterinary Medicine, Istanbul University, Avcilar 34320, Istanbul, Turkey. bozkurt@istanbul.edu.tr
- 2Leather Technology Programe, Vocational School of Technical Sciences, Istanbul University, Avcilar 34320, Istanbul, Turkey.
- KMID: 1090794
- DOI: http://doi.org/10.4142/jvs.2007.8.3.219
Abstract
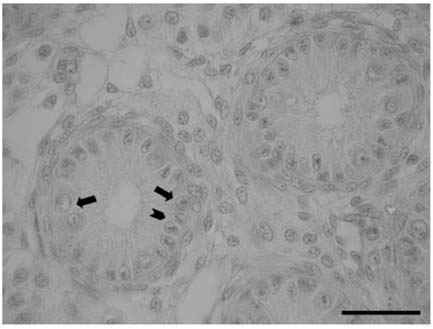
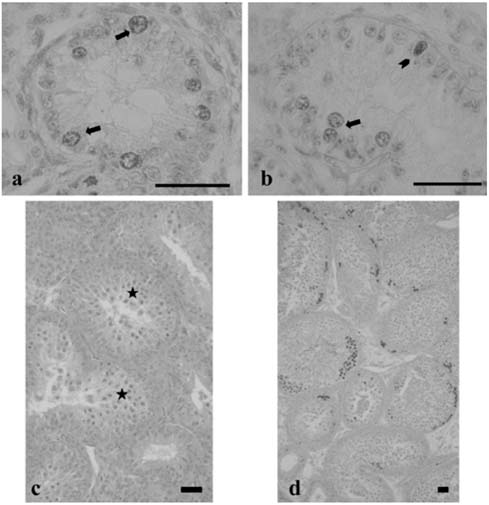
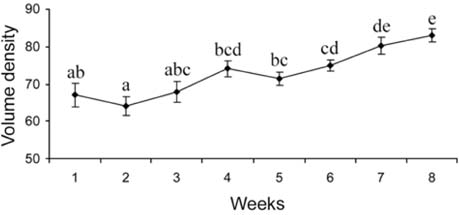
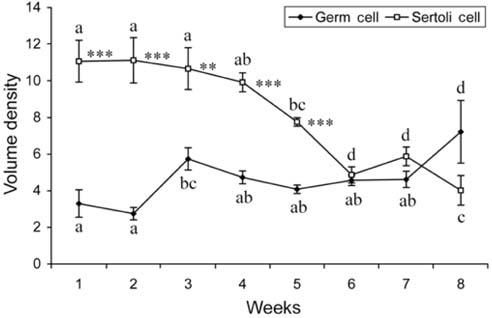
- There has been no study aimed at directly determiningof the periods of Sertoli cell proliferation in birds evendomestic fowl. The aims of this study were to observe thecessation of post-hatching mitotic proliferation of Sertolicells in domestic fowl, and to determine the volumedensity of Sertoli and germ cells during this period. Atotal of 50 Leghorn chicks were used in this study. Thetestes sections of the animals were immunostained withBrdU to observe the proliferation of cells from one to 10weeks of age. The volume density of the Sertoli and germcells were determined using the standard point countingmethod. The volume density of the germ cell nuclei wasinitially less than that of the Sertoli cells but the volumedensity converged by week 6, and remained relativelyconstant until the commencement of meiosis. Clearlabeling of Sertoli and germ cells was observed from week1 to week 7. The only those cells still labeled after 8 weekswere germ cells, indicating that Sertoli cell proliferationhad ceased. Therefore, it is recommended that anyresearch into the testes of domestic fowl should considerthe cessation of Sertoli cell proliferation by approximately8 weeks.
Keyword
MeSH Terms
Figure
Reference
-
1. Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogen on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000. 141:3898–3907.
Article2. Atanassova NN, Walker M, McKinnell C, Fisher JS, Sharpe RM. Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol. 2005. 184:107–117.
Article3. Berndtson WE, Igboeli G, Parker WG. The numbers of Sertoli cells in mature Holstein bulls and their relationship to quantitative aspects of spermatogenesis. Biol Reprod. 1987. 37:60–67.
Article4. De Reviers M, Hochereau de Reviers MT, Blanc MR, Brillard JP, Courot M, Pelletier J. Control of Sertoli and germ cell populations in the cock and sheep testis. Reprod Nutr Dev. 1980. 20:241–249.
Article5. De Reviers M, Williams JB. Cunningham FJ, Lake PE, Hewitt D, editors. Testis development and production of spermatozoa in the cockerel (Gallus Dometicus). Reproductive Biology of Poultry. 1984. Harlow: British Poultry Science;183–202.6. Isobe N, Shimada M. Effects of estrone sulfate administration on reproductive function in male Japanese quail. J Poult Sci. 2003. 40:247–253.
Article7. Kirby D, Froman DP. Whittow GC, editor. Reproduction in Male birds. Sturkie's Avian Physiology. 2000. 5th ed. London: Academic Press;597–615.
Article8. Maeda T, Yoshimura Y. Effects of diethylstilbestrol administration on sperm motility and reproductive function in male Japanese quail (Coturnix japonica). J Poult Sci. 2002. 39:27–33.
Article9. McCoard SA, Lunstra DD, Wise TH, Ford JJ. Specific staining of Sertoli cell nuclei and evaluation of Sertoli cell number and proliferative activity in meishan and white composite boars during the neonatal period. Bio Reprod. 2001. 64:689–695.
Article10. McGuinness MP, Orth JM. Reinitiation of gonocyte mitosis and movement of gonocytes to the basement membrane in testes of newborn rats in vivo and in vitro. Anat Rec. 1992. 233:527–537.
Article11. Meehan T, Schlatt S, O'Bryan MK, de Kretser DM, Loveland KL. Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH. Dev Bio. 2000. 220:225–237.
Article12. Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982. 203:485–492.
Article13. Orth JM, Gunsalus GM, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid numbers in adults depends on number of Sertoli cells produced during perinatal development. Endocrinology. 1988. 122:787–794.
Article14. Russell LD, Ettlin R, Hikim APS, Clegg ED, editors. Tissue preperation for evaluation of testis. Histological and Histopathological Evaluation of the Testis. 1990. Clearwater: Cache River Press;195–209.15. Sharpe RM. Knobil E, Neill JD, editors. Regulation of spermatogenesis. Physiology of Reproduction. 1994. New York: Raven Press;1363–1434.16. Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003. 125:769–784.
Article17. Sharpe RM, Walker M, Millar MR, Atanassova N, Morris K, McKinnell C, Saunders PTK, Fraser HM. Effect of neonatal gonadotropin-releasing hormone antagonist administration on Sertoli cell number and testicular development in the marmoset: comparison with the rat. Biol Reprod. 2000. 62:1685–1693.
Article18. Sprando RL, Russell LD. Spermiogenesis in the red-ear turtle (Pseudemys scripta) and the domestic fowl (Gallus domesticus): a study of cytoplasmic events including cell volume changes and cytoplasmic elimination. J Morphol. 1988. 198:95–118.
Article