J Vet Sci.
2006 Sep;7(3):217-223. 10.4142/jvs.2006.7.3.217.
Protective effect of the isoflavone equol against DNA damage induced by ultraviolet radiation to hairless mouse skin
- Affiliations
-
- 1Department of Veterinary Pathology, Faculty of Veterinary Medicine, Gadjah Mada University, Jl. Olah Raga, Karang Malang, Yogyakarta, Indonesia. sitarina_id@yahoo.com.au
- KMID: 1089904
- DOI: http://doi.org/10.4142/jvs.2006.7.3.217
Abstract
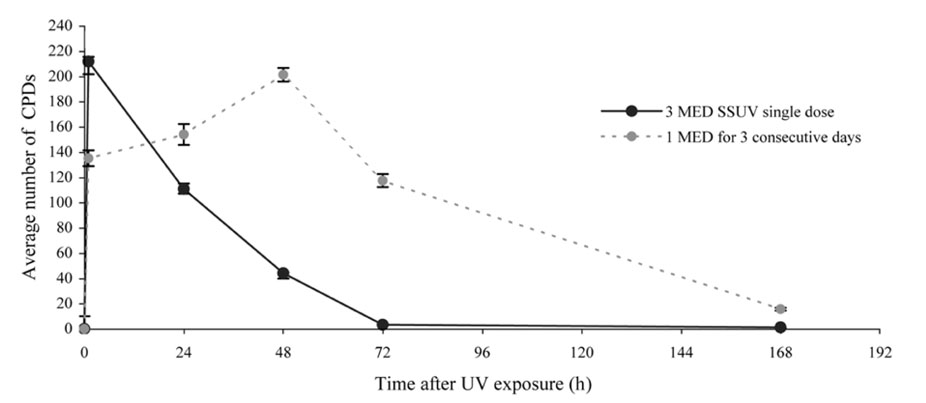
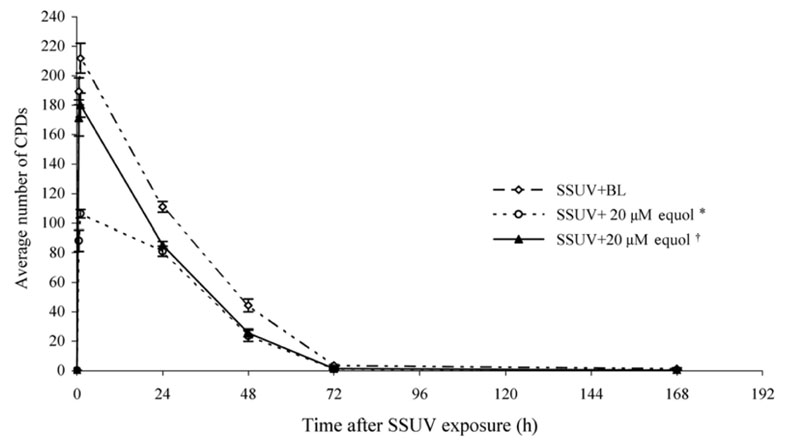
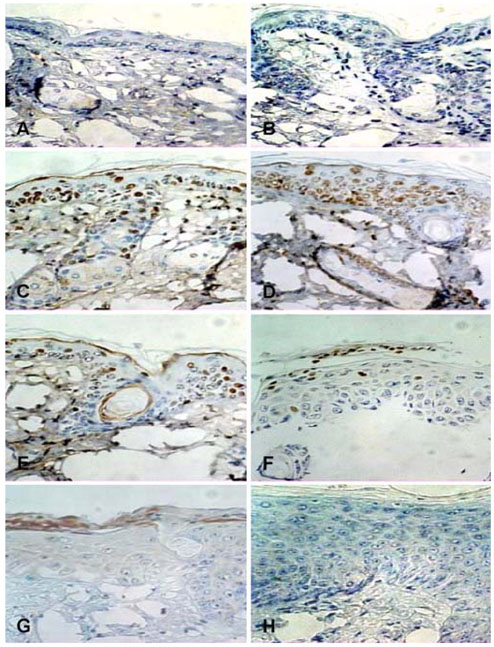
- Equol, an isoflavonoid metabolite produced from the dietary isoflavone daidzein by the gut microflora in mammals, has been found to protect not only against ultraviolet (UV) radiation-induced cutaneous inflammation and photoimmune suppression, but also have antiphotocarcinogenic properties in mice. Because the state of DNA damage has been correlated with suppression of the immune system and photocarcinogenesis, we have therefore examined the potential of equol to offer protection from solar-simulated UV (SSUV) radiation-induced DNA damage in hairless mice by the immunohistochemical approach using monoclonal antibody specific for cyclobutane pyrimidine dimers (CPDs; H3 antibody). Topical application of 20 micrometer equol lotion, which was applied both before and after SSUV significantly reduced the number of CPDs. This reduction was evident immediately after SSUV exposure, at 1 h after exposure, and at 24 h after exposure, revealing 54%, 50%, and 26% reduction in CPDs, respectively. When the same concentration was applied for 5 consecutive days after SSUV exposure, there was no significant difference in the reduction of CPDs immediately after SSUV irradiation or at 1 hour afterwards, but there were significant reductions of 23% and 42% at 24 and 48 h after SSUV exposure, respectively. Despite apparently reducing the number of CPDs post-SSUV, topically applied equol did not appear to increase the rate of dimer removal. To conclude, equol applied topically prior to SSUV irradiation offers protection against CPD formation in hairless mice, possibly by acting as a suncreen and thus inhibiting DNA photodamage.
Keyword
MeSH Terms
Figure
Reference
-
1. Ananthaswamy HN, Loughlin SM, Cox P, Evans RL, Ullrich SE, Kripke ML. Sunlight and skin cancer: inhibition of p53 mutations in UV-irradiated mouse skin by sunscreens. Nature Med. 1997. 3:510–514.
Article2. Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989. 170:1117–1131.
Article3. Berg RJ, Ruven HJ, Sands AT, de Gruijl FR, Mullenders LH. Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J Invest Dermatol. 1998. 110:405–409.
Article4. Berneburg M, Krutmann J. Photoimmunology, DNA repair and photocarcinogenesis. J Photochem Photobiol B. 2000. 54:87–93.
Article5. Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991. 88:10124–10128.
Article6. Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996. 1:136–142.7. Caceres-Dittmar G, Ariizumi K, Xu S, Tapia FJ, Bergstresser PR, Takashima A. Hydrogen peroxide mediates UV-induced impairment of antigen presentation in a murine epidermal-derived dendritic cell line. Photochem Photobiol. 1995. 62:176–183.
Article8. Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993. 261:1442–1445.
Article9. Franke AA, Custer LJ, Cerna CM, Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc Soc Exp Biol Med. 1995. 208:18–26.
Article10. Garssen J, Vandebriel RJ, van Loveren H. Molecular aspects of UVB-induced immunosuppression. Arch Toxicol Suppl. 1997. 19:97–109.11. Gibbs NK, Norval M, Traynor NJ, Wolf M, Johnson BE, Crosby J. Action spectra for the trans to cis photoisomerisation of urocanic acid in vitro and in mouse skin. Photochem Photobiol. 1993. 57:584–590.
Article12. Greinert R, Boguhn O, Harder D, Breitbart EW, Mitchell DL, Volkmer B. The dose dependence of cyclobutane dimer induction and repair in UVB-irradiated human keratinocytes. Photochem Photobiol. 2000. 72:701–708.
Article13. Kraemer KH. Sunlight and skin cancer: another linked revealed. Proc Natl Acad Sci USA. 1997. 94:11–14.14. Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992. 89:7516–7520.
Article15. Kripke ML. Immunologic mechanisms in UV radiation carcinogenesis. Adv Cancer Res. 1981. 34:69–106.
Article16. Ley RD, Fourtanier A. Sunscreen protection against ultraviolet radiation-induced pyrimidine dimers in mouse epidermal DNA. Photochem Photobiol. 1997. 65:1007–1011.
Article17. Ley RD. Photoreactivation of UV-induced pyrimidine dimers and erythema in the marsupial Monodelphis domestica. Proc Natl Acad Sci USA. 1985. 82:2409–2411.
Article18. Mohammad T, Tessman I, Morrison H, Kennedy MA, Simmonds SW. Photosensitized inactivation of infectious DNA by urocanic acid, indoleacrylic acid and rhodium complexes. Photochem Photobiol. 1994. 59:189–196.
Article19. Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, Kripke ML. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc Natl Acad Sci USA. 1996. 93:10354–10359.
Article20. Noonan FP, De Fabo EC. Immunosuppression by ultraviolet B radiation: initiation by urocanic acid. Immunol Today. 1992. 13:250–254.
Article21. Qin X, Zhang S, Oda H, Nakatsuru Y, Shimizu S, Yamazaki Y, Nikaido O, Ishikawa T. Quantitative detection of ultraviolet light-induced photoproducts in mouse skin by immunohistochemistry. Jpn J Cancer Res. 1995. 86:1041–1048.
Article22. Roza L, De Gruijl FR, Bergen Henegouwen JB, Guikers K, Van Weelden H, Van Der Schans GP, Baan RA. Detection of photorepair of UV-induced thymine dimers in human epidermis by immunofluorescence microscopy. J Invest Dermatol. 1991. 96:903–907.
Article23. Simon MM, Aragane Y, Schwarz A, Luger TA, Schwarz T. UVB light induces nuclear factor kappa B (NF kappa B) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J Invest Dermatol. 1994. 102:422–427.
Article24. Strickland PT. Distribution of thymine dimers induced in mouse skin by ultraviolet radiation. Photodermatol. 1988. 5:1–8.25. Urbach F. Ultraviolet radiation and skin cancer of humans. J Photochem Photobiol B. 1997. 40:3–7.
Article26. Vink AA, Berg RJ, de Gruijl FR, Roza L, Baan RA. Induction, repair and accumulation of thymine dimers in the skin of UV-B-irradiated hairless mice. Carcinogenesis. 1991. 12:861–864.
Article27. Widyarini S, Allanson M, Gallagher NL, Pedley J, Boyle GM, Parsons PG, Whiteman DC, Walker C, Reeve VE. Isoflavonoid photoprotection in mouse and human skin is dependent on metallothionein. J Invest Dermatol. 2006. 126:198–204.
Article28. Widyarini S, Husband AJ, Reeve VE. Protective effect of the isoflavonoid equol against hairless mouse skin carcinogenesis induced by UV radiation alone or with a chemical cocarcinogen. Photochem Photobiol. 2005. 81:32–37.
Article29. Widyarini S, Spinks N, Husband AJ, Reeve VE. Isoflavonoid compounds from red clover (Trifolium pratense) protect from inflammation and immune suppression induced by UV radiation. Photochem Photobiol. 2001. 74:465–470.
Article30. Wolf P, Yarosh DB, Kripke ML. Effects of sunscreens and a DNA excision repair enzyme on ultraviolet radiation-induced inflammation, immune suppression, and cyclobutane pyrimidine dimer formation in mice. J Invest Dermatol. 1993. 101:523–527.
Article31. Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994. 372:773–776.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Ultraviolet Light Damage in Mammalian Cells by Flowcytometry
- Galangin (3,5,7-Trihydroxyflavone) Shields Human Keratinocytes from Ultraviolet B-Induced Oxidative Stress
- Photoprotective Effect of Various Sunscreens Against Ultraviolet B - induced Chronic Skin Damage
- Rosmarinic Acid Inhibits Ultraviolet B-Mediated Oxidative Damage via the AKT/ERK-NRF2-GSH Pathway In Vitro and In Vivo
- The protective effects of trace elements against side effects induced by ionizing radiation