J Vet Sci.
2006 Dec;7(4):369-374. 10.4142/jvs.2006.7.4.369.
Detection of betanodaviruses in apparently healthy aquarium fishes and invertebrates
- Affiliations
-
- 1KRF Zoonotic Disease Priority Research Institute, Seoul National University, Seoul 151-742, Korea. parksec@snu.ac.kr
- 2Department of Veterinary Medicine, College of Applied Life Sciences, Cheju National University, Jeju 690-756, Korea.
- 3Faculty of Marine Technology, Chonnam National University, Yeosu 550-749, Korea.
- 4College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- KMID: 1089485
- DOI: http://doi.org/10.4142/jvs.2006.7.4.369
Abstract
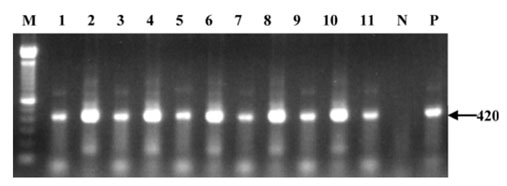
- Betanodaviruses are the causative agents of viral nervous necrosis (VNN) in cultured marine fish. A total of 237 apparently healthy aquarium fish, marine (65 species) and freshwater (12 species) fishes and marine invertebrates (4 species), which were stocked in a commercial aquarium in Seoul, South Korea, were collected from November 2005 to February 2006. The brains of the fish and other tissues of the invertebrates were examined by reverse transcriptasepolymerase chain reaction (RT-PCR) and nested PCR to detect betanodavirus. Positive nested PCR results were obtained from the brains of 8 marine fish species (shrimp fish Aeoliscus strigatus, milkfish Chanos chanos, three spot damsel Dascyllus trimaculatus, Japanese anchovy Engraulis japonicus, pinecone fish Monocentris japonica, blue ribbon eel Rhinomuraena quaesita, look down fish Selene vomer, yellow tang Zebrasoma flavesenes), 1 marine invertebrate species (spiny lobster Pamulirus versicolor), and 2 freshwater fish species (South American leaf fish Monocirrhus polyacanthus and red piranha Pygocentrus nattereri). The detection rate in nested PCR was 11/237 (4.64%). These subclinically infected aquarium fish and invertebrates may constitute an inoculum source of betanodaviruses for cultured fishes in the Korean Peninsula.
Keyword
MeSH Terms
Figure
Reference
-
1. Arcier JM, Herman F, Lightner DV, Redman RM, Mari J, Bonami JR. A viral disease associated with mortalities in hatchery-reared postlarvae of the giant freshwater prawn Macrobrachium rosenbergii. Dis Aquat Organ. 1999. 38:177–181.
Article2. Arimoto M, Mushiake K, Mizuta Y, Nakai T, Muroga K, Furusawa I. Detection of striped jack nervous necrosis virus (SJNNV) by enzyme-linked immunosorbent assay (ELISA). Fish Pathol. 1992. 27:191–195.
Article3. Barker DE, MacKinnon AM, Boston L, Burt MDB, Cone DK, Speare DJ, Griffiths S, Cook M, Ritchie R, Olivier G. First report of piscine nodavirus infecting wild winter flounder Pleuronectes americanus in Passamaquoddy Bay, New Brunswick, Canada. Dis Aquat Organ. 2002. 49:99–105.
Article4. Breuil G, Pépin JFP, Boscher S, Thiéry R. Experimental vertical transmission of nodavirus from broodfish to eggs and lavae of the sea bass, Dicentrachus labrax (L.). J Fish Dis. 2002. 25:697–702.
Article5. Castric J, Thiéry R, Jeffroy J, de Kinkelin P, Raymond JC. Sea bream Sparus aurata, an asymptomatic contagious fish host for nodavirus. Dis Aquat Organ. 2001. 47:33–38.
Article6. Chang SF, Ngoh GH, Kueh S. Detection of viral nervous necrosis nodavirus by reverse transcription polymerase chain reaction in locally farmed marine food fish. Singapore Vet J. 1997. 21:39–44.7. Chi SC, Lo J, Lin SC, Wen WW, Lo GF, Kou GH, Chen SN. Bondad-Reantaso MG, Humphery S, Kanchanakan S, Chinabut S, editors. The survey of viral nervous necrosis among cultured groupers in Taiwan. APEC FWG 02/2000. Development of a Regional Research Program on Grouper Virus Transmission and Vaccine Development. 2000. Bangkok: Asia-Pacific Economic Cooperation;58–61.8. Chi SC, Shieh JR, Lin SJ. Genetic and antigenic analysis of betanodaviruses isolated from aquatic organisms in Taiwan. Dis Aquat Organ. 2003. 55:221–228.
Article9. Curtis PA, Drawbridge M, Iwamoto T, Nakai T, Hedrick RP, Gendron AP. Nodavirus infection of juvenile white seabass, Atractoscion nobilis, cultured in southern California: first record of viral nervous necrosis (VNN) in North America. J Fish Dis. 2001. 24:263–271.
Article10. Gomez DK, Sato J, Mushiake K, Isshiki T, Okinaka Y, Nakai T. PCR-based detection of betanodaviruses from cultured and wild marine fish with no clinical signs. J Fish Dis. 2004. 27:603–608.
Article11. Hedge A, Teh HC, Lam TJ, Sin YM. Nodavirus infection in freshwater ornamental fish, guppy Poicelia reticulata - comparative characterization and pathogenicity studies. Arch Virol. 2003. 148:575–586.12. Kim JH, Hayward CJ, Heo GJ. Nematode worm infections (Camallanus cotti; Camallanidae) in guppies (Poecilia reticulata) imported to Korea. Aquaculture. 2002. 205:231–235.
Article13. Kim JH, Hayward CJ, Joh SJ, Heo GJ. Parasitic infections in live freshwater tropical fishes imported to Korea. Dis Aquat Organ. 2002. 52:169–173.
Article14. Kim SR, Jung SJ, Kim YJ, Kim JD, Jung TS, Choi TJ, Yoshimizu M, Oh MJ. Phylogenetic comparison of viral nervous necrosis (VNN) viruses occurring seed production period. J Korean Fish Soc. 2002. 35:237–241.
Article15. Lim MGB, Chong SY, Kueh S. Some observations of grouper (Epinephelus tauvina) and seabass (Lates calcarifer) nodavirus infection in Singapore. Singapore Vet J. 1997. 21:47–51.16. Mori K, Nakai T, Muroga K, Arimoto M, Mushiake K, Furusawa I. Properties of a new virus belonging to nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology. 1992. 187:368–371.
Article17. Munday BL, Kwang J, Moody N. Betanodavirus infections of teleost fish: a review. J Fish Dis. 2002. 25:127–142.
Article18. Muroga K. Viral and bacterial diseases in larval and juvenile marine fish and shellfish: a review. Fish Pathol. 1995. 30:71–85.
Article19. Muroga K. Viral and bacterial diseases of marine fish and shellfish in Japanese hatcheries. Aquaculture. 2001. 202:23–44.
Article20. Office International des epizooties OIE. International aquatic animal health code. Manual of Diagnostic Tests for Aquatic Animals. 1997. Paris: OIE;267.21. Office International des epizooties OIE. Viral encephalopathy and retinopathy. Manual of Diagnostic Tests for Aquatic Animals. 2003. Paris: OIE;135–141.22. Oh MJ, Jung SJ, Kim SR, Rajendran KV, Kim YJ, Choi TJ, Kim HR, Kim JD. A fish nodavirus associated with mass mortality in hatchery-reared red drum, Sciaenops ocellatus. Aquaculture. 2002. 211:1–7.
Article23. Sohn SG, Park MA, Lee SD, Chun SK. Studies on the mass mortality of the cultured grouper, Epinephelus septemfasciatus. J Fish Pathol. 1991. 4:87–94.24. Sohn SG, Park MA, Oh MJ, Chun SK. A fish nodavirus isolated cultured sevenband grouper Epinephelus septemfasciatus. J Fish Pathol. 1998. 11-2:97–104.25. Sri Widada J, Durand S, Cambournac I, Qian D, Shi Z, Dejonghe E, Richard V, Bonami JR. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii: dot-blot, in situ hybridization and RT-PCR. J Fish Dis. 2003. 26:583–590.
Article26. Watanabe K, Nishizawa T, Yoshimizu M. Selection of broodstock candidates of barfin flounder using an ELISA system with recombinant protein of barfin flounder nervous necrosis virus. Dis Aquat Organ. 2000. 41:219–223.
Article27. Yoshikoshi K, Inoue K. Viral nervous necrosis in hatchery-reared larvae and juveniles of Japanese parrotfish, Oplegnathus fasciatus (Temminck & Schlegel). J Fish Dis. 1990. 13:69–77.
Article28. Zafran , Harada T, Koesharyani I, Yuasa K, Hatai K. Indonesian hatchery reared seabass larvae (Lates calcarifer) associated with viral nervous necrosis (VNN). Indonesian Fish Res J. 1998. 4:19–22.
Article29. Zafran , Koesharyani I, Johnny F, Yuasa K, Harada T, Hatai K. Viral nervous necrosis in humpback grouper Cromileptes altivelis larvae and juveniles in Indonesia. Fish Pathol. 2000. 35:95–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Analytical Data of the Traditional Foods in Korea: I. A Cholesterol Content of Fishes and Shell Fishes
- Distribution of cestodes in the digestive tract of Indian Hill-stream fishes
- Isolation and identification of Vibrio harveyi from chub mackerel (Scomber japonicus)
- Parasitic Diseases caused by Fishes Populary Eaten Raw
- Isolation of a zoonotic pathogen Aeromonas hydrophila from freshwater stingray (Potamotrygon motoro) kept in a Korean aquarium with ricefish (Oryzias latipes)