J Vet Sci.
2006 Dec;7(4):343-348. 10.4142/jvs.2006.7.4.343.
In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood
- Affiliations
-
- 1Laboratory of Stem cell and Tumor Biology, Department of Veterinary Public Health, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. kangpub@snu.ac.kr, leeys@snu.ac.kr
- 2Adult Stem Cell Research Center, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- KMID: 1089481
- DOI: http://doi.org/10.4142/jvs.2006.7.4.343
Abstract
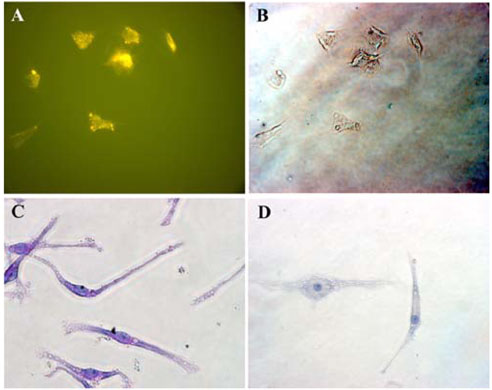
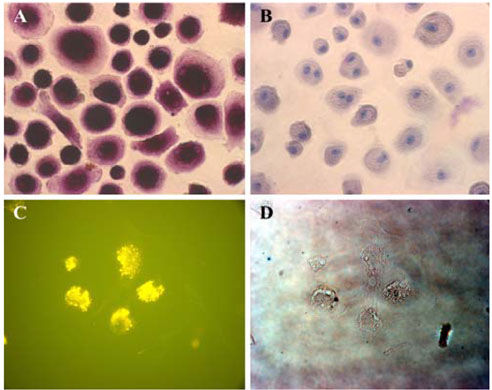
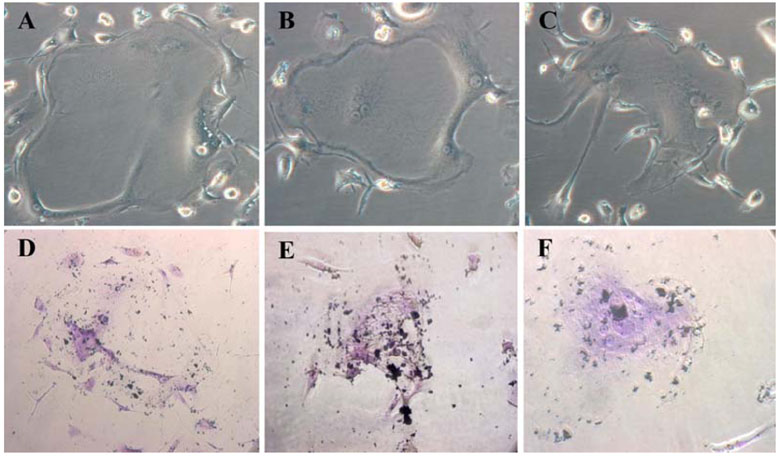
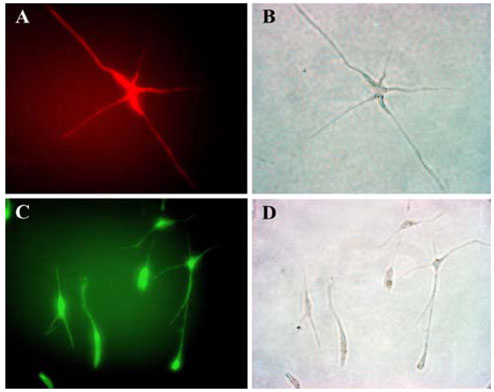
- Mesenchymal stem cells (MSCs) have the capabilities for self-renewal and differentiation into cells with the phenotypes of bone, cartilage, neurons and fat cells. These features of MSCs have attracted the attention of investigators for using MSCs for cell-based therapies to treat several human diseases. Because bone marrowderived cells, which are a main source of MSCs, are not always acceptable due to a significant drop in their cell number and proliferative/differentiation capacity with age, human umbilical cord blood (UCB) cells are good substitutes for BMCs due to the immaturity of newborn cells. Although the isolation of hematopoietic stem cells from UCB has been well established, the isolation and characterization of MSCs from UCB still need to be established and evaluated. In this study, we isolated and characterized MSCs. UCB-derived mononuclear cells, which gave rise to adherent cells, exhibited either an osteoclast or a mesenchymal-like phenotype. The attached cells with mesenchymal phenotypes displayed fibroblast-like morphologies, and they expressed mesenchymal-related antigens (SH2 and vimentin) and periodic acid Schiff activity. Also, UCB-derived MSCs were able to transdifferentiate into bone and 2 types of neuronal cells, in vitro. Therefore, it is suggested that the MSCs from UCB might be a good alternative to bone marrow cells for transplantation or cell therapy.
MeSH Terms
Figure
Cited by 3 articles
-
Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs
Ji-Hey Lim, Ye-Eun Byeon, Hak-Hyun Ryu, Yun-Hyeok Jeong, Young-Won Lee, Wan Hee Kim, Kyung-Sun Kang, Oh-Kyeong Kweon
J Vet Sci. 2007;8(3):275-282. doi: 10.4142/jvs.2007.8.3.275.Enhanced tyrosine hydroxylase expression in PC12 cells co-cultured with feline mesenchymal stem cells
Guang-Zhen Jin, Xi-Jun Yin, Xian-Feng Yu, Su-Jin Cho, Hyo-Sang Lee, Hyo-Jong Lee, Il-Keun Kong
J Vet Sci. 2007;8(4):377-382. doi: 10.4142/jvs.2007.8.4.377.Regenerative Cell Therapy for the Sensorineural Hearing Loss
Kyoung Ho Park
Hanyang Med Rev. 2015;35(2):113-120. doi: 10.7599/hmr.2015.35.2.113.
Reference
-
1. Bendall LJ, Kortlepel K, Gottlieb DJ. Human acute myeloid leukemia cells bind to bone marrow stroma via a combination of beta-1 and beta-2 integrin mechanisms. Blood. 1993. 82:3125–3132.
Article2. Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989. 86:3828–3832.
Article3. Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999. 181:67–73.
Article4. D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999. 14:1115–1122.5. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article6. Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993. 82:66–76.
Article7. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, Cooper S, English D, Kurtzberg J, Bard J, Boyse EA. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989. 321:1174–1178.
Article8. Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, Fernandez M, Chastang C. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997. 337:373–381.
Article9. Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001. 7:581–588.
Article10. Gutierrez-Rodriguez M, Reyes-Maldonado E, Mayani H. Characterization of the adherent cells developed in Dexter-type long-term cultures from human umbilical cord blood. Stem Cells. 2000. 18:46–52.
Article11. Han IS, Ra JS, Kim MW, Lee EA, Jun HY, Park SK, Kwon BS. Differentiation of CD34+ cells from human cord blood and murine bone marrow is suppressed by C6 beta-chemokines. Mol Cells. 2003. 15:176–180.12. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.
Article13. Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, Yasumizu T, Teraoka H. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003. 21:217–227.
Article14. Kim SK, Koh SK, Song SU, Shin SH, Choi GS, Kim WC, Lee MH, Seoh JY, Park SK, Fraser JK. Ex vivo expansion and clonality of CD34+ selected cells from bone marrow and cord blood in a serum-free media. Mol Cells. 2002. 14:367–373.15. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004. 103:1669–1675.
Article16. Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001. 86:1099–1100.17. Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999. 48:913–927.
Article18. Peault B. Hematopoietic stem cell emergence in embryonic life: developmental hematology revisited. J Hematother. 1996. 5:369–378.
Article19. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article20. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003. 21:105–110.
Article21. Roux S, Quinn J, Pichaud F, Orcel P, Chastre E, Jullienne A, De Vernejoul MC. Human cord blood monocytes undergo terminal osteoclast differentiation in vitro in the presence of culture medium conditioned by giant cell tumor of bone. J Cell Physiol. 1996. 168:489–498.
Article22. Shields LE, Andrews RG. Gestational age changes in circulating CD34+ hematopoietic stem/progenitor cells in fetal cord blood. Am J Obstet Gynecol. 1998. 178:931–937.
Article23. Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992. 13:66–80.
Article24. Tavassoli M, Minguell JJ. Homing of hemopoietic progenitor cells to the marrow. Proc Soc Exp Biol Med. 1991. 196:367–373.
Article25. Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990. 87:7260–7264.
Article26. Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003. 121:368–374.
Article27. Wyrsch A, dalle Carbonare V, Jansen W, Chklovskaia E, Nissen C, Surbek D, Holzgreve W, Tichelli A, Wodnar-Filipowicz A. Umbilical cord blood from preterm human fetuses is rich in committed and primitive hematopoietic progenitors with high proliferative and self-renewal capacity. Exp Hematol. 1999. 27:1338–1345.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Difference in Cell Characteristics among the Monoclonal Cell Populations Obtained from the Human Umbilical Cord Blood Derived Mesenchymal Stem Cell Population