J Vet Sci.
2006 Dec;7(4):339-342. 10.4142/jvs.2006.7.4.339.
Site adaptations of Acanthogyrus (Acanthosentis) tilapiae: Observations through light and scanning electron microscopy
- Affiliations
-
- 1Hydrobiology Department, National Research Center, Giza, Egypt. bayoumy2004@yahoo.com
- KMID: 1089480
- DOI: http://doi.org/10.4142/jvs.2006.7.4.339
Abstract
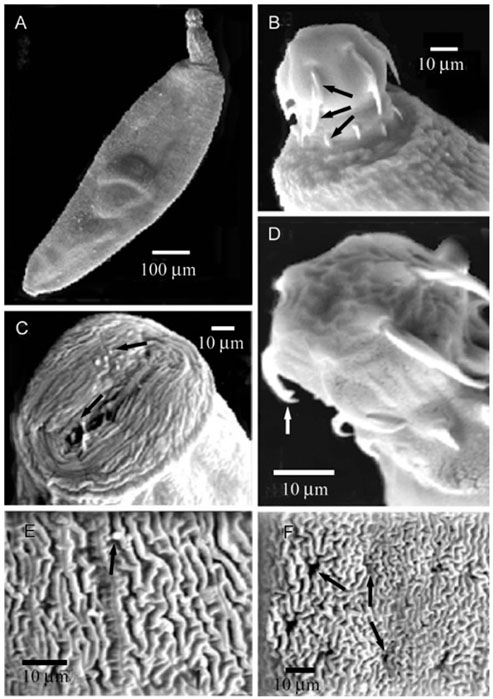
- Acanthogyrus (Acanthosentis) tilapiae parasites were collected from the intestines of 300 fish belonging to three tilapia species sourced at the River Nile, Giza, Egypt. The proboscis of the parasite was characterized by three rows of hooks that curved towards the posterior of the body. The first row is supported by unmodified hooks. The parasite tegument has a series of alternative folds and a large number of pores. Sensory ganglia are located on the surface of the proboscis and body. Acanthogyrus (Acanthosentis) tilapiae provokes an aggressive host response indicated by hyperplasia of the intestinal goblet cells and focal eosinophil infiltrations. This acanthocephalan parasite shows a highly modified adaptation to its site of host infection.
Keyword
MeSH Terms
Figure
Reference
-
1. Alava JJ, Aguirre WE. Scanning electron microscopy of Neoechinorhynchus sp. (Acanthocephala: Neoechinorhynchidae), a possible new species of intestinal parasite of the Tallfin croaker Micropogonias altipinnis (Günther, 1864). Parasitol Latinoam. 2005. 60:48–53.2. Ali MA, Abdel-Baki AS, Sakran T, Entzeroth R, Abdel-Ghaffar F. Light and electron microscopic studies of Myxobolus stomum n. sp. (Myxosporea: Myxobolidae) infecting the blackspotted grunt Plectorhynicus gaterinus (Forsskal, 1775) in the Red Sea, Egypt. Parasitol Res. 2003. 91:390–397.3. Amin OM. Crompton DWT, Nickol BB, editors. Classification. Biology of the Acanthocephala. 1985. Cambridge: Cambridge University Press;27–72.4. Amin OM. Occurrence of the subgenus Acanthosentis Verma & Datta, 1929 (Acanthocephala: Quadrigyridae) in Japan, with the description of Acanthogyrus (Acanthosentis) alternatspinus n. sp. and A. (A.) parareceptaclis n. sp. from Lake Biwa drainage fishes and a key to the species of the subgenus. Syst Parasitol. 2005. 60:125–137.
Article5. Amin OM, Heckmann RA. Description and pathology of Neoechinorhynchus idahoensis n. sp. (Acanthocephala: Neoechinorhynchidae) in Catostomus columbianus from Idaho. J Parasitol. 1992. 78:34–39.
Article6. Amin OM, Redlin MJ. The effect of host species on growth and variability of Echinorhynchus salmonis Müller, 1784 (Acanthocephala: Echinorhynchidae), with special reference to the status of the genus. Syst Parasitol. 1980. 2:9–20.
Article7. Baylis HA. A new acanthocephalan from an East African freshwater fish. Ann Mag Nat Hist. 1947. 14:861–868.8. Bayoumy EM, El-Monem SA, Hassanain MA. Ultrastructural observations of spermatogenesis and spermiogenesis in the fish-gill fluke, Pseudohaliotrema plectocirra Paperna, 1972 (Ancyrocephalinae, Monogenea). J Egypt Vet Med. 2005. 65:259–267.9. Carleton HM. Carleton's Histological Technique. 1980. 5th ed. New York: Oxford University Press;71–95.10. Dezfuli BS, Giari L, Simoni E, Bosi G, Manera M. Histopathology, immunohistochemistry and ultrastructure of the intestine of Leuciscus cephalus (L.) naturally infected with Pomphorhynchus laevis (Acanthocephala). J Fish Dis. 2002. 25:7–14.
Article11. El-Monem SA, Bayoumy ME, Hassanain MA. Descreption of five new myxosporean parasites infecting some Red Sea fishes in Egypt. Egypt J Zool. 2005. 45:333–348.12. El-Naggar MM, El-Abbassy SA. Anatomical observations on the viviparous monogenean Gyrodactylus rysavyi Ergens, 1973 from the Nile catfish Clarias gariepinus in Egypt. Egypt J Zool. 2003. 40:225–249.13. El-Naggar MM, El-Naggar AM, El-Abbassy SA. Microhabitat and movement of the vivipraous monogeneans Gyrodactylus alberti, Macrogyrodactylus clarii and M. congolensis from the Nile catfish Clarias gariepinus. J Egypt Ger Soc Zool Invertebr Zool Parasitol. 2001. 35:169–187.14. El-Naggar MM, Hagras AE, Ogawa K, Hussien AB, El-Naggar AM. A correlation between heavy metals in water and the gills of Oreochromis niloticus and Tilapia zilli and the intensity of their parasitic monogeneans at Manzala lake and the River Nile in Egypt. J Egypt Ger Soc Zool Invertebr Zool Parasitol. 2000. 32:189–204.15. El-Naggar MM, Serag HM. Redescription of Macrogyrodactylus clarii Gussev 1961, a monogenean gill parasite of Clarias lazera in Egypt. Arab Gulf J Sci Res Agric Biol Sci. 1987. 5:257–271.16. Esch GW, Huffines WJ. Histopathology associated with endoparasitic helminths in bass. J Parasitol. 1973. 59:306–313.
Article17. Golvan YJ. Le phylum des Acanthocephala. Deuxième note. La classe de Eoacanthocephala (Van Cleave 1936). Ann Parasitol Hum Comp. 1959. 34:5–52.
Article18. Halton DW. The surface topography of a monogenean, Diclidophora merlangi revealed by scanning electron microscopy. Z Parasitenkd. 1979. 61:1–12.
Article19. Martins ML. Doenças infecciosas e parasitárias de peixes. Boletim Técnico Caunesp n. 3. 1998. 2nd ed. Jaboticabal: Funesp;66.20. Mehlhorn H. Encyclopedic Reference of Parasitology: Diseases, Treatment, Therapy. 2001. 2nd ed. Berlin: Springer;10–25.21. Miller DM, Dunagan TT. Body wall organization of the Acanthocephalan, Macracanthorhynchus hirudinaceus: a reexamination of the lacunar system. Proc Helminthological Soc Wash. 1976. 43:99–106.22. Petrochenko VI. Acanthocephala of Domestic and Wild Animals. 1971. Vol. 1. Jerusalem: Israel Program for Scientific Translations;435.23. Ramadan EI, Shalby SI, Imam EA, Abu-Elezz NMT. Studies on some gastrointestinal helminth parasites of freshwater fish in Egypt. I. Prevalence. Bull NR C Egypt. 1989. 14:143–152.24. Ramasamy P, Brennan GP. Ultrastructure of the surface structures and haptor of Empleurosoma pyriforme (Ancyrocephalinae; Monopisthocotylea: Monogenea) from the gills of the teleost fish Therapon jarbua. Parasitol Res. 2000. 86:129–139.
Article25. Taraschewski H. Host-parasite interactions in Acanthocephala: a morphological approach. Adv Parasitol. 2000. 46:1–179.
Article26. Yamaguti S. Systema Helminthum. 1963. Vol. 5. New York: Interscience Publishers;423.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morpholgical Study of Korean Pubic Louse , Phthirus pubis ( Linnaeus , 1758 ) by Light and Scanning Electron Microscopy
- Recent advances in electron microscopy for the diagnosis and research of glomerular diseases
- Scanning electron microscopic study of capillary change in bleomycin-induced pulmonary fibrosis
- The genus Hypoxylon, Wood Decay Fungi - II. Teleomorph of Annulata Section
- Histological Characteristics of the Interface of Corneal Stroma and Descemet's Membrane