J Vet Sci.
2006 Dec;7(4):333-337. 10.4142/jvs.2006.7.4.333.
Pharmacokinetics, urinary excretion and dosage regimen of levofloxacin following a single intramuscular administration in cross bred calves
- Affiliations
-
- 1Department of Pharmacology and Toxicology, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Science University, Ludhiana-141004, India. vkdumka@yahoo.com
- 2Faculty of Veterinary Science and Animal Husbandry, Sher-e-Kashmir University of Agricultural Science and Technology, R S Pura, Jammu-181102, India.
- KMID: 1089479
- DOI: http://doi.org/10.4142/jvs.2006.7.4.333
Abstract
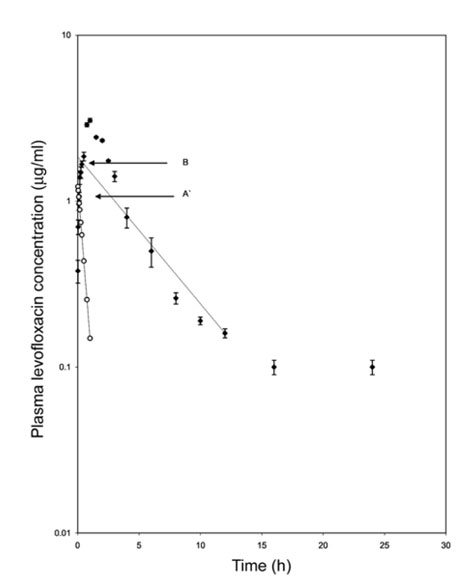
- The pharmacokinetics and urinary excretion following single intramuscular administration of levofloxacin at a dose of 4 mg/kg was investigated in seven male cross bred calves. Appreciable plasma concentration of levofloxacin (0.38 +/- 0.06 microgram/ml) was detected at 1 min after injection and the peak plasma level of 3.07 +/- 0.08 microgram/ml was observed at 1 h. The drug level above MIC(90) in plasma was detected up to 12 h after administration. Rapid absorption of the drug was also evident by the high value of the absorption rate constant (2.14 +/- 0.24 /h). The overall systemic bioavailability of levofloxacin, after intramuscular administration, was 56.6 +/- 12.4%. The high value of AUC (7.66 +/- 0.72 mg.h/ml) reflected the vast area of body covered by drug concentration. Extensive distribution of the drug into various body fluids and tissues was noted by the high value of Vd(area) (1.02 +/- 0.05 l/kg). The high ratio of AUC/MIC (76.6 +/- 7.25) obtained in this study indicated excellent clinical and bacteriological efficacy of levofloxacin in calves. The elimination half-life and MRT were 3.67 +/- 0.4 h and 5.57 +/- 0.51 h, respectively. The total body clearance (Cl(B)) was 204.9 +/- 22.6 ml/kg/h. On the basis of the pharmacokinetic parameters, a suitable intramuscular dosage regimen for levofloxacin in calves would be 1.5 mg/kg repeated at 12 h intervals.
Keyword
MeSH Terms
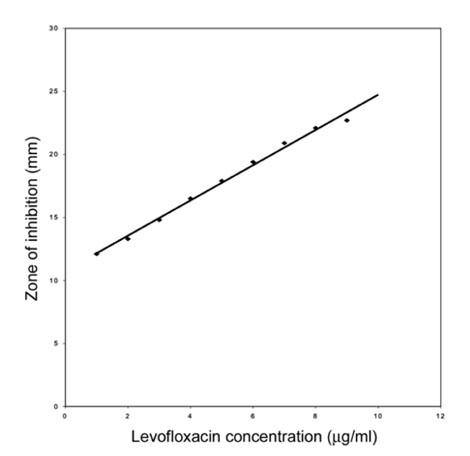
Figure
Cited by 1 articles
-
Disposition kinetics and dosage regimen of levofloxacin on concomitant administration with paracetamol in crossbred calves
Vinod K. Dumka
J Vet Sci. 2007;8(4):357-360. doi: 10.4142/jvs.2007.8.4.357.
Reference
-
1. Aliabadi FS, Lees P. Pharmacokinetics and pharmacodynamics of danofloxacin in serum and tissue fluids of goats following intravenous and intramuscular administration. Am J Vet Res. 2001. 62:1979–1989.
Article2. Apley MD, Upson DW. Lung tissue concentrations and plasma pharmacokinetics of danofloxacin in calves with acute pneumonia. Am J Vet Res. 1993. 54:937–943.3. Arret B, Johnson DP, Kirshbaum A. Outline of details for microbiological assays of antibiotics: second revision. J Pharm Sci. 1971. 60:1689–1694.
Article4. Atef M, El-Gendi AY, Aziza , Amer MM, El-Aty AM. Some pharmacokinetic data for danofloxacin in healthy goats. Vet Res Commun. 2001. 25:367–377.5. Baggot JD. Principles of Drug Disposition in Domestic Animals. 1977. Philadelphia: Saunders;190–218.6. Bakken JS. The fluoroquinolones: how long will their utility last? Scand J Infect Dis. 2004. 36:85–92.
Article7. Chulavatnatol S, Chindavijak B, Vibhagool A, Wananukul W, Sriapha C, Sirisangtragul C. Pharmacokinetics of levofloxacin in healthy Thai male volunteers. J Med Assoc Thai. 1999. 82:1127–1135.8. Davis R, Bryson HM. Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994. 47:677–700.9. Edelstein PH, Edelstein MA, Lehr KH, Ren J. In-vitro activity of levofloxacin against clinical isolates of Legionella spp, its pharmacokinetics in guinea pigs, and use in experimental Legionella pneumophila pneumonia. J Antimicrob Chemother. 1996. 37:117–126.
Article10. Gibaldi M, Perrier D. Pharmacokinetics. 1982. 2nd ed. New York: Marcel Dekker;433–444.11. Giles CJ, Magonigle RA, Grimshaw WTR, Tanner AC, Risk JE, Lynch MJ, Rice JR. Clinical pharmacokinetics of parenterally administered danofloxacin in cattle. J Vet Pharmacol Ther. 1991. 14:400–410.
Article12. Gips M, Soback S. Norfloxacin nicotinate pharmacokinetics in unweaned and weaned calves. J Vet Pharmacol Ther. 1996. 19:130–134.
Article13. Kaartinen L, Salonen M, Alli L, Pyorala S. Pharmacokinetics of enrofloxacin after single intravenous, intramuscular and subcutaneous injections in lactating cows. J Vet Pharmacol Ther. 1995. 18:357–362.
Article14. Klesel N, Geweniger KH, Koletzki P, Isert D, Limbert M, Markus A, Riess G, Schramm H, Iyer P. Chemotherapeutic activity of levofloxacin (HR 355, DR-3355) against systemic and localized infections in laboratory animals. J Antimicrob Chemother. 1995. 35:805–819.
Article15. Kunin CM, Dornbush AC, Finland M. Distribution and excretion of four tetracycline analogues in normal young men. J Clin Invest. 1959. 38:1950–1963.
Article16. Langtry HD, Lamb HM. Levofloxacin. Its use in infections of the respiratory tract, skin, soft tissues and urinary tract. Drugs. 1998. 56:487–515.17. North DS, Fish DN, Redington JJ. Levofloxacin, a second-generation fluoroquinolone. Pharmacotherapy. 1998. 18:915–935.18. Schneider M, Valle M, Woehrle F, Boisrame B. Pharmacokinetics of marbofloxacin in lactating cows after repeated intramuscular administrations and pharmacodynamics against mastitis isolated strains. J Dairy Sci. 2004. 87:202–211.
Article19. Shem-Tov M, Ziv G, Glickman A, Saran A. Pharmacokinetics and penetration of marbofloxacin from blood into the milk of cows and ewes. Zentralbl Veterinarmed A. 1997. 44:511–519.
Article20. Shojaee Aliabadi F, Landoni MF, Lees P. Pharmacokinetics (PK), pharmacodynamics (PD) and PK-PD integration of danofloxacin in sheep biological fluids. Antimicrob Agents Chemother. 2003. 47:626–635.
Article21. Singh K, Srivastava AK. Plasma levels, pharmacokinetics, urinary excretion and dosage regimen of ciprofloxacin in cross bred cow calves. J Punjab Acad Sci. 2000. 2:105–107.22. TerHune TN, Skogerboe TL, Shostorm VK, Weigel DJ. Comparison of pharmacokinetics of danofloxacin and enrofloxacin in calves challenged with Mannheimia haemolytica. Am J Vet Res. 2005. 66:342–349.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disposition kinetics and dosage regimen of levofloxacin on concomitant administration with paracetamol in crossbred calves
- Subcutaneous pharmacokinetics and dosage regimen of cefotaxime in buffalo calves (Bubalus bubalis)
- Disposition kinetics and urinary excretion of cefpirome after intravenous injection in buffalo calves
- Pharmacokinetics and dosage regimen of ceftriaxone in E. coli lipopolysaccharide induced fever in buffalo calves
- Effect of fever on pharmacokinetics and dosage regimen of intramuscularly administered amikacin in goats