J Vet Sci.
2009 Mar;10(1):9-13. 10.4142/jvs.2009.10.1.9.
Distribution and ontogeny of gastrin- and serotonin-immunoreactive cells in the proventriculus of developing chick, Gallus gallus domestica
- Affiliations
-
- 1Histology and Pathology Laboratory, Mediterranean Fisheries Research Production and Education Institute, PK 190, Antalya, 07001, Turkey. aksoy@akdenizsuurunleri.gov.tr
- 2Department of Biology, Suleyman Demirel University, Isparta, 32260, Turkey.
- KMID: 1089340
- DOI: http://doi.org/10.4142/jvs.2009.10.1.9
Abstract
- The ontogeny and distribution of gastrin- and serotonin-immunoreactive (IR) cell in the proventriculus of chicks (Gallus gallus domestica, n = 60) in different growth periods was examined immunohistochemically using antisera specific to gastrin and serotonin. Gastrin and serotonin-IR cells were detected in chick proventriculus. Gastrin-IR cells were first evident after 12 days of incubation in lamina epithelialis and compound glands, while serotonin-IR cells were observed only in compound glands at that same time. Gastrin-IR and serotonin-IR cells increased in frequency on incubation day 14 and 16, respectively. Towards the end of incubation, gastrin- and serotonin-IR cell numbers decreased. In adult chicken, both IR cells were present but not lower numbers. The observations demonstrate the presence of gastrin- and serotonin-IR cells in the proventriculus of developing chicks in temporally changing frequencies.
Keyword
MeSH Terms
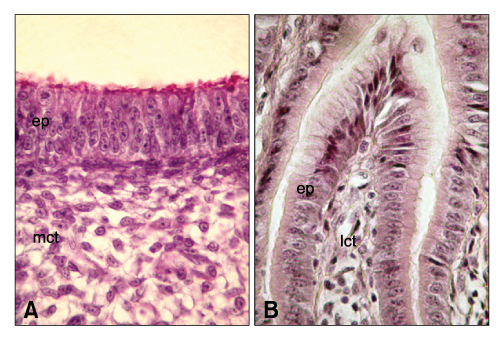
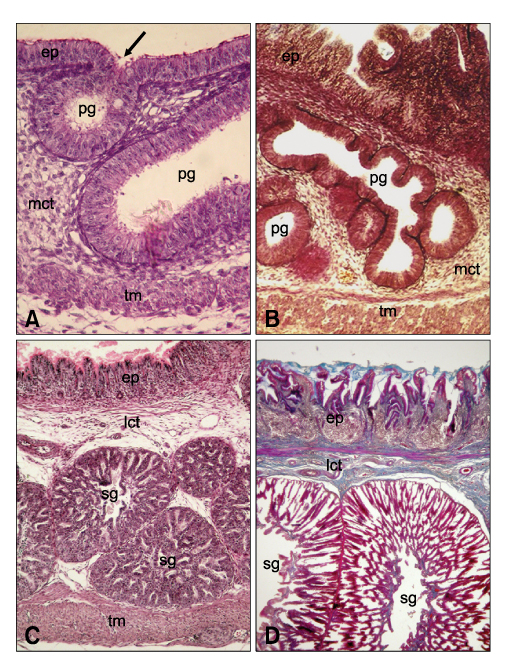
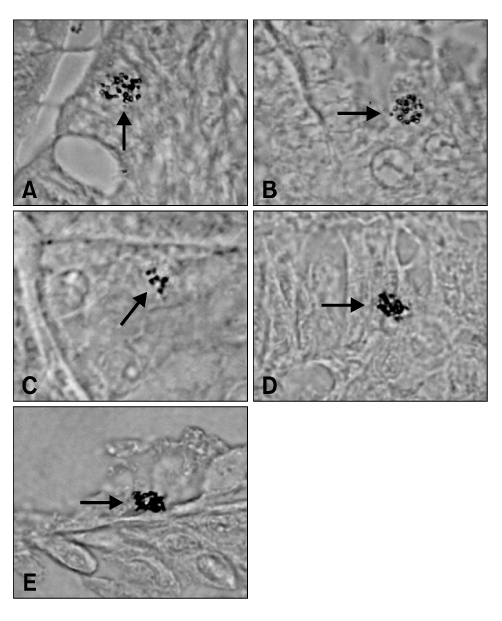
Figure
Reference
-
1. Alison BC. The distribution and ontogeny of gastrin/CCK-, somatostatin- and neurotensin-immunoreactive cells in the gastrointestinal tract of the chicken. Histol Histopathol. 1989. 4:55–62.2. Alison BC. The ontogeny and distribution of glucagon- and pancreatic polypeptide-immunoreactive cells in the gastrointestinal tract of the chicken. Anat Embryol. 1990. 182:605–610.
Article3. Beorlegui C, Martínez A, Sesma P. Endocrine cells and nerves in the pyloric ceca and the intestine of Oncorhynchus mykiss (Teleostei): An immunocytochemical study. Gen Comp Endocrinol. 1992. 86:483–495.
Article4. Castaldo L, Lucini C. An immunohistochemical study on the endocrine cells in the gastrointestinal tract of domestic duck. Eur J Basic Appl Histochem. 1991. 35:131–143.5. Castaldo L, Lucini C. Ontogenesis of some endocrine cells in the duck gastrointestinal tract. Eur J Histochem. 1994. 38:319–326.6. Catroxo MHB, Lima MAI, Petrella S. Ultrastructure of endocrine cells of the stomach (proventriculus and gizzard) of the red-capped cardinal (Paroaria g. gularis, Linnaeus, 1766). Rev Chil Anat. 2001. 19:239–244.7. D'este L, Biancone S, Renda T. Ontogenesis of 5-hydroxytryptamine-like immunoreactive cells and their relationship with bombesin in chicken proventriculus. Basic Appl Histochem. 1986. 30:109–117.8. Dimaline R, Lee CM. Chicken Gastrin: A member of the gastrin/CCK family with novel structure-activity relationships. Am J Physiol. 1990. 259:G882–G888.
Article9. Dockray GJ. Gastrin and gastric epithelial physiology. J Physiol. 1999. 518:315–324.10. Gulmez N, Nazli M, Aslan S, Liman N. Immunolocalisation of serotonin, gastrin, somatostatin and glucagon in enteroendocrine cells of the goose (Anser anser). Acta Vet Hung. 2003. 51:439–449.
Article11. Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981. 29:577–580.
Article12. Larsson LI. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc Res Tech. 2000. 48:272–281.
Article13. Lee HS, Ku SK. An immunohistochemical study of endocrine cells in the alimentary tract of the grass lizard, Takydromus wolteri Fischer (Laceridae). Acta Histochem. 2004. 106:171–178.
Article14. Lee JH, Ku SK, Lee HS, Kitagawa H. An immunohistochemical study of endocrine cells in the pancreas of the Red-bellied frog (Bombina orientalis). Eur J Histochem. 2003. 47:165–172.
Article15. Luna LG. Carson FL, editor. Masson trichrome stain. Histotechnology: A Self Instructional Text. 1997. Chicago: American Society for Clinical Pathology Press;134.16. Martínez A, López J, Barrenechea MA, Sesma P. Immunocytochemical and ultrastructural characterization of endocrine cells in chicken proventriculus. Cell Tissue Res. 1991. 263:541–548.
Article17. Martínez A, López J, Sesma P. Development of the diffuse endocrine system in the chicken proventriculus. Cell Tissue Res. 1993. 271:107–113.
Article18. Martínez A, López J, Sesma P. The nervous system of the chicken proventriculus: an immunocytochemical and ultrastructural study. Histochem J. 2000. 32:63–70.19. Mensah-Brown EPK, Lawrence PA. Neurotransmitters regulating acid secretion in the proventriculus of the houbara bustard (Chlamydotis undulata): A morphological viewpoint. J Morphol. 2001. 248:175–184.
Article20. Neglia S, Arcamone N, Esposito V, Gargiulo G, De Girolamo P. Presence and distribution of ghrelin-immunopositive cells in the chicken gastrointestinal tract. Acta Histochem. 2005. 107:3–9.
Article21. Okamoto T, Fujii S. An electron microscopic study on endocrine cells in the pyloric region of the duck. Nippon Juigaku Zasshi. 1980. 42:169–176.
Article22. Peroutka SJ. 5-Hydroxytryptamine receptor subtypes. Annu Rev Neurosci. 1988. 11:45–60.
Article23. Rawdon BB. Gastrointestinal hormones in birds: morphological, chemical, and developmental aspects. J Exp Zool. 1984. 232:659–670.
Article24. Rawdon BB, Andrew A. An immunocytochemical survey of endocrine cells in the gastrointestinal tract of chicks at hatching. Cell Tissue Res. 1981. 220:279–292.
Article25. Rawdon BB, Andrew A. Distribution of serotonin-immunoreactive gut endocrine cells in chicks at hatching. examination of possible co-localisation with peptides reveals unexpected cross-reactivity of substance P antiserum with serotonin. Histochemistry. 1994. 102:93–100.
Article26. Rawdon BB, Andrew A. Gut endocrine cells in birds: an overview, with particular reference to the chemistry of gut peptides and the distribution, ontogeny, embryonic origin and differentiation of the endocrine cells. Prog Histochem Cytochem. 1999. 34:3–82.
Article27. Richardson BP, Engel G. The pharmacology and function of 5-HT3 receptors. Trends Neurosci. 1986. 9:424–428.28. Salvi E, Vaccaro R, Renda TG. An immunohistochemical study of the ontogeny of the neuroendocrine system in the chicken oesophagus. Anat Embryol. 1998. 197:283–291.
Article29. Timurkaan S, Karan M, Aydin A. Immunohistochemical study of the distribution of serotonin in the gastrointestinal tract of the porcupines (Hystrix cristata). Rev Med Vet. 2005. 156:533–536.30. Yamaguchi S, Yamada J, Kitamura N, Yamashita T. Ontogeny of the endocrine cells in the quail proventriculus. Z Mikrosk Anat Forsch. 1986. 100:981–989.31. Yaman M, Tarakçi BG, Bayrakdar A, Yaman I. Immunohistochemical study of the endocrine cells in the oesophagus of the ostrich (Struthio camelus). Rev Med Vet. 2008. 159:63–67.32. Yamanaka Y, Yamada J, Kitamura N, Yamashita T. An immunohistochemical study on the distribution of endocrine cells in the chicken gastrointestinal tract. Z Mikrosk Anat Forsch. 1989. 103:437–446.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A new cestode Raillietina (Skrjabinia) doggaddaensis n. sp. from Gallus gallus domesticus (L.) from India
- Immunohistochemical Studies on the Serotonin, Somatostatin and Gastrin-Positive Cells in the Gastric Adenocarcinoma
- Morphological Changes of Endolymphatic Sac after Gentamicin Injection to Chick Embryo
- Regional Distribution and Relative Frequency of Gastrointestinal Endocrine Cells in Large Intestines of C57BL/6 Mice
- Immunocytochemical study on the somatostatin,serotonin and gastrin cells in the gastrointestinal tract of the percida