Korean J Radiol.
2009 Apr;10(2):112-120. 10.3348/kjr.2009.10.2.112.
SPIO-Enhanced MRI Findings of Well-Differentiated Hepatocellular Carcinomas: Correlation with MDCT Findings
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. kshyun@skku.edu
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- KMID: 1088721
- DOI: http://doi.org/10.3348/kjr.2009.10.2.112
Abstract
OBJECTIVE
This study was designed to assess superparamagnetic iron oxide (SPIO)-enhanced MRI findings of well-differentiated hepatocellular carcinomas (HCCs) correlated with their multidetector-row CT (MDCT) findings.
MATERIALS AND METHODS
Seventy-two patients with 84 pathologically proven well-differentiated HCCs underwent triple-phase MDCT and SPIO-enhanced MRI at a magnetic field strength of 1.5 Tesla (n = 49) and 3.0 Tesla (n = 23). Two radiologists in consensus retrospectively reviewed the CT and MR images for attenuation value and the signal intensity of each tumor. The proportion of hyperintense HCCs as depicted on SPIO-enhanced T2- or T2*-weighted images were compared in terms of tumor size (< 1 cm and > 1 cm), five CT attenuation patterns based on arterial and equilibrium phases and magnetic field strength, by the use of univariate and multivariate analyses.
RESULTS
Seventy-eight (93%) and 71 (85%) HCCs were identified by CT and on SPIO-enhanced T2- and T2*-weighted images, respectively. For the CT attenuation pattern, one (14%) of seven isodense-isodense, four (67%) of six hypodense-hypodense, four (80%) of five isodense-hypodense, 14 (88%) of 16 hyperdense-isodense and 48 (96%) of 50 hyperdense-hypodense HCCs were hyperintense (Cochran-Armitage test for trend, p < 0.001). Based on the use of multivariate analysis, the CT attenuation pattern was the only factor that affected the proportion of hyperintense HCCs as depicted on SPIO-enhanced T2- or T2*-weighted images (p < 0.001). Tumor size or magnetic field strength was not a factor that affected the proportion of hyperintense HCCs based on the use of univariate and multivariate analysis (p > 0.05).
CONCLUSION
Most well-differentiated HCCs show hyperintensity on SPIO-enhanced MRI, although the lesions show various CT attenuation patterns. The CT attenuation pattern is the main factor that affects the proportion of hyperintense well-differentiated HCCs as depicted on SPIO-enhanced MRI.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Carcinoma, Hepatocellular/*pathology
Contrast Media
Female
Humans
Image Enhancement/methods
Image Processing, Computer-Assisted
Iron/*diagnostic use
Liver Neoplasms/*pathology
Magnetic Resonance Imaging/*methods
Male
Middle Aged
Oxides/*diagnostic use
Retrospective Studies
Tomography, X-Ray Computed/*methods
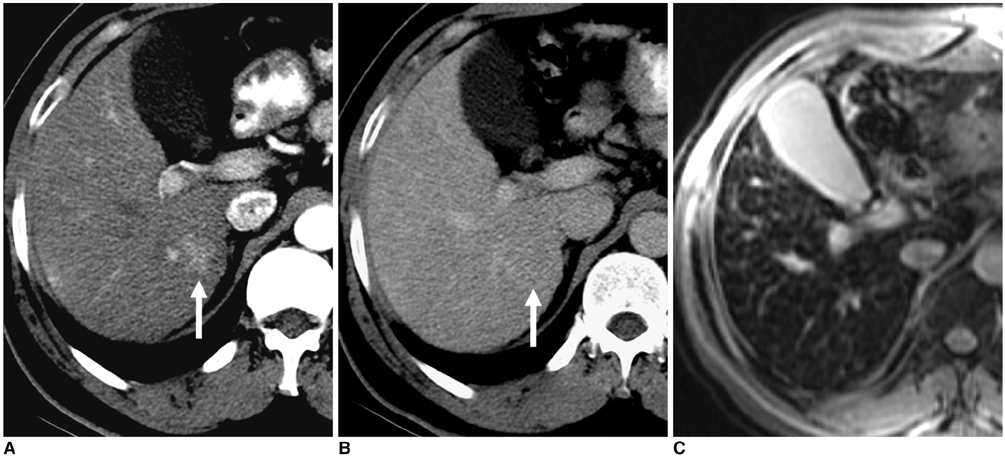
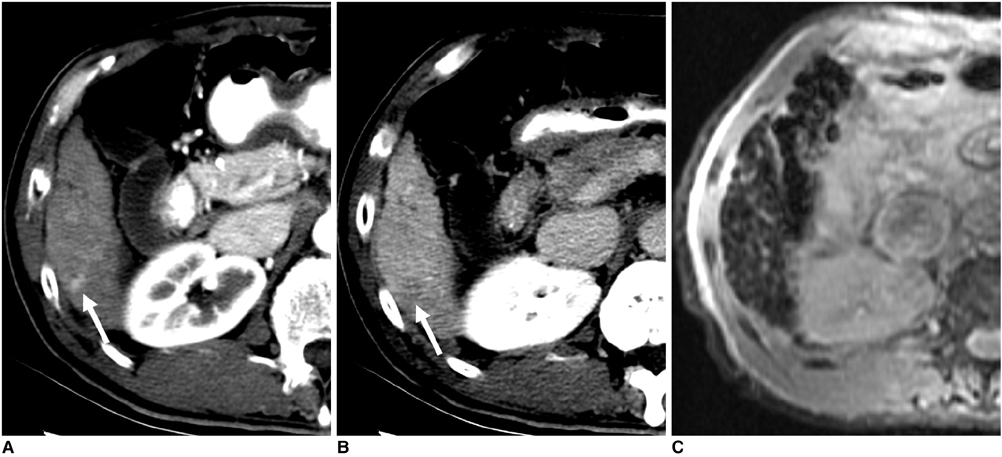
Figure
Reference
-
1. Hussain SM, Zondervan PE, IJzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002. 22:1023–1036.2. Lim JH, Kim MJ, Park CK, Kang SS, Lee WJ, Lim HK. Dysplastic nodules in liver cirrhosis: detection with triple phase helical dynamic CT. Br J Radiol. 2004. 77:911–916.3. Figueras J, Jaurrieta E, Valls C, Ramos E, Serrano T, Rafecas A, et al. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy. J Am Coll Surg. 2000. 190:580–587.4. Lim JH, Choi D, Kim SH, Lee SJ, Lee WJ, Lim HK, et al. Detection of hepatocellular carcinoma: value of adding delayed phase imaging to dual-phase helical CT. AJR Am J Roentgenol. 2002. 179:67–73.5. Iannaccone R, Laghi A, Catalano C, Rossi P, Mangiapane F, Murakami T, et al. Hepatocellular carcinoma: role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology. 2005. 234:460–467.6. Li CS, Chen RC, Tu HY, Shih LS, Zhang TA, Lii JM, et al. Imaging well-differentiated hepatocellular carcinoma with dynamic triple-phase helical computed tomography. Br J Radiol. 2006. 79:659–665.7. Amano S, Ebara M, Yajima T, Fukuda H, Yoshikawa M, Sugiura N, et al. Assessment of cancer cell differentiation in small hepatocellular carcinoma by computed tomography and magnetic resonance imaging. J Gastroenterol Hepatol. 2003. 18:273–279.8. Takayasu K, Muramatsu Y, Mizuguchi Y, Moriyama N, Ojima H. Imaging of early hepatocellular carcinoma and adenomatous hyperplasia (dysplastic nodules) with dynamic CT and a combination of CT and angiography: experience with resected liver specimens. Intervirology. 2004. 47:199–208.9. Murakami T, Hori M, Kim T, Kawata S, Abe H, Nakamura H. Multidetector row CT and MR imaging in diagnosing hepatocellular carcinoma. Intervirology. 2004. 47:209–226.10. Sakabe K, Yamamoto T, Kubo S, Hirohashi K, Hamuro M, Nakamura K, et al. Correlation between dynamic computed tomographic and histopathological findings in the diagnosis of small hepatocellular carcinoma. Dig Surg. 2004. 21:413–420.11. Tanaka M, Nakashima O, Wada Y, Kage M, Kojiro M. Pathomorphological study of Kupffer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology. 1996. 24:807–812.12. Ishida T, Murakami T, Kato N, Takahashi M, Miyazawa T, Tsuda K, et al. Superparamagnetic iron oxide enhanced magnetic resonance imaging of rat liver with hepatocellular carcinoma and benign hyperplastic nodule. Invest Radiol. 1997. 32:282–287.13. Imai Y, Murakami T, Yoshida S, Nishikawa M, Ohsawa M, Tokunaga K, et al. Superparamagnetic iron oxide-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histologic grading. Hepatology. 2000. 32:205–212.14. Kim SH, Choi D, Kim SH, Lim JH, Lee WJ, Kim MJ, et al. Ferucarbotran-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2005. 184:1069–1076.15. Kato H, Kanematsu M, Kondo H, Goshima S, Matsuo M, Hoshi H, et al. Ferumoxide-enhanced MR imaging of hepatocellular carcinoma: correlation with histologic tumor grade and tumor vascularity. J Magn Reson Imaging. 2004. 19:76–81.16. Lim JH, Choi D, Cho SK, Kim SH, Lee WJ, Lim HK, et al. Conspicuity of hepatocellular nodular lesions in cirrhotic livers at ferumoxides-enhanced MR imaging: importance of Kupffer cell number. Radiology. 2001. 220:669–676.17. Pauleit D, Textor J, Bachmann R, Conrad R, Flacke S, Layer G, et al. Hepatocellular carcinoma: detection with gadolinium- and ferumoxides-enhanced MR imaging of the liver. Radiology. 2002. 222:73–80.18. Tang Y, Yamashita Y, Arakawa A, Namimoto T, Mitsuzaki K, Abe Y, et al. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium- and ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 1999. 172:1547–1554.19. Matsuo M, Kanematsu M, Itoh K, Ito K, Maetani Y, Kondo H, et al. Detection of malignant hepatic tumors: comparison of gadolinium- and ferumoxide-enhanced MR imaging. AJR Am J Roentgenol. 2001. 177:637–643.20. Nakamura H, Ito N, Kotake F, Mizokami Y, Matsuoka T. Tumor-detecting capacity and clinical usefulness of SPIO-MRI in patients with hepatocellular carcinoma. J Gastroenterol. 2000. 35:849–855.21. Bartolozzi C, Crocetti L, Lencioni R, Cioni D, Della Pina C, Campani D. Biliary and reticuloendothelial impairment in hepatocarcinogenesis: the diagnostic role of tissue-specific MR contrast media. Eur Radiol. 2007. 17:2519–2530.22. Takeshita K, Nagashima I, Frui S, Takada K, Yamauchi T, Harasawa A, et al. Effect of superparamagnetic iron oxide-enhanced MRI of the liver with hepatocellular carcinoma and hyperplastic nodule. J Comput Assist Tomogr. 2002. 26:451–455.23. Kang BK, Lim JH, Kim SH, Choi D, Lim HK, Lee WJ, et al. Preoperative depiction of hepatocellular carcinoma: ferumoxides-enhanced MR imaging versus triple-phase helical CT. Radiology. 2003. 226:79–85.24. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954. 7:462–503.25. Kim SH, Lim HK, Choi D, Lee WJ, Kim SH, Kim MJ, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: effect of histologic grade on therapeutic results. AJR Am J Roentgenol. 2006. 186:S327–S333.26. Efremidis SC, Hytiroglou P, Matsui O. Enhancement patterns and signal-intensity characteristics of small hepatocellular carcinoma in cirrhosis: pathologic basis and diagnostic challenges. Eur Radiol. 2007. 17:2969–2982.27. Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999. 172:969–976.28. Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991. 22:172–178.29. International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995. 22:983–993.30. Sugihara S, Nakashima O, Kojiro M, Majima Y, Tanaka M, Tanikawa K. The morphologic transition in hepatocellular carcinoma: a comparison of the individual histologic features disclosed by ultrasound-guided fine-needle biopsy with those of autopsy. Cancer. 1992. 70:1488–1492.31. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.32. Chang JM, Lee JM, Lee MW, Choi JY, Kim SH, Lee JY, et al. Superparamagnetic iron oxide-enhanced liver magnetic resonance imaging: comparison of 1.5 T and 3.0 T imaging for detection of focal malignant liver lesions. Invest Radiol. 2006. 41:168–174.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Ferucarbotran-Enhanced MRI and Triple-Phase MDCT for the Detection of Hepatocellular Carcinoma in Advanced Liver Cirrhosis
- Detectability of Hepatocellular Carcinoma: Comparison of Gd-DT PA-Enhanced and SPIO-Enhanced MR Imaging
- The Clinical Usefulness of SPIO-MRI in Detection and Staging of Hepatocellular Carcinoma
- Evaluation of Fibrosis in Liver Cirrhosis by Superparamagnetic Iron Oxide (SPIO)-Enhanced MR Imaging: Does the Radiological Non-Invasive Fibrosis Index Correlate with the Laboratory Non-Invasive Fibrosis Index?
- Detection of Hepatic Metastases from Colorectal Cancer: A Comparative Study between the SPIO-enhanced MR and Intraoperative Ultrasound