Yonsei Med J.
2008 Apr;49(2):279-287. 10.3349/ymj.2008.49.2.279.
Secretion of Biologically Active Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor by Transduced Gastric Cancer Cells
- Affiliations
-
- 1Department of Microbiology, Ajou University School of Medicine, Suwon, Korea. kimkm@ajou.ac.kr
- KMID: 1084502
- DOI: http://doi.org/10.3349/ymj.2008.49.2.279
Abstract
- PURPOSE
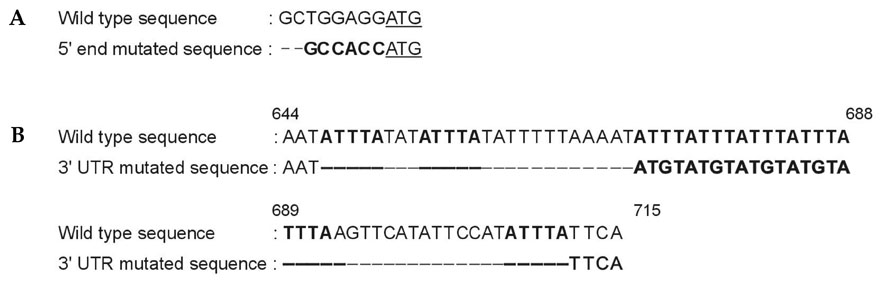
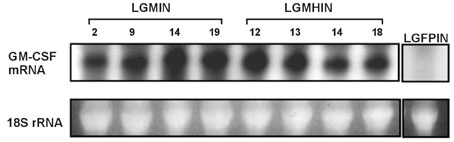
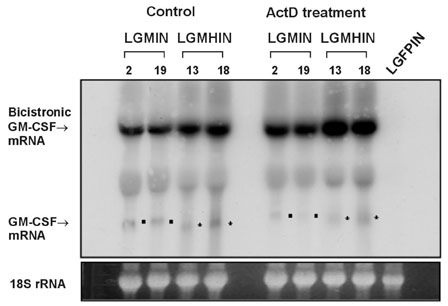
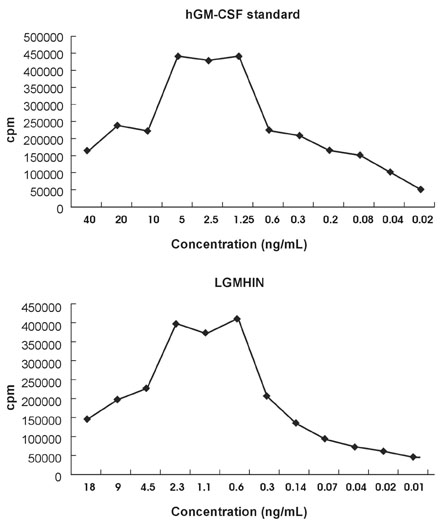
Gastric cancer has the highest incidence rate among cancers in Asia. The advanced type of signet ring cell carcinoma has poor prognosis compared to other types of gastric cancer. The immuno-gene therapy with cytokine-based tumor vaccines has not yet been investigated for gastric cancer. The granulocyte macrophage colony-stimulating factor (GM-CSF)-based tumor vaccine has been demonstrated as the most potent stimulator for specific and long-lasting systemic tumor immunity. MATERIALS AND METHODS: In the present study, KATO III cells, the human signet ring cell gastric carcinoma cell line, were genetically modified by the transduction with the human GM-CSF cDNA or the modified hGM-CSF in replication-deficient retroviruses. The genomic integrations and mRNA expressions of the transgenes were determined by Southern and Northern blot analyses. RESULTS: Wild type (wt) or modified hGM-CSF was integrated into the genome of KATO III cells. The modified hGM-CSF mRNA was more stable than that of wt. The KATO III cells with the modified hGM-CSF produced higher level of hGM-CSF (12.4-19 ng/10(6)cells/48hrs) than that with wt hGM-CSF, when determined by enzyme-linked immunosorbent assay (ELISA). The secreted recombinant hGM-CSF could support the proliferation of the GM-CSF-dependent cell line, indicating that the hGM-CSF secreted by the transduced KATO III cells has biological activities. Irradiated, transduced KATO III cells continued to secret hGM-CSF without proliferation. CONCLUSION: Our results suggest that GM-CSF secreting KATO III cells could be tested for the treatment of gastric cancer as an allogeneic tumor vaccine as a part of immunotherapeutic treatment.
Keyword
MeSH Terms
-
Base Sequence
Blotting, Northern
Blotting, Southern
Cell Line, Tumor
Enzyme-Linked Immunosorbent Assay
Granulocyte Macrophage Colony-Stimulating Factors,
Humans
Mutagenesis
RNA, Messenger/genetics/metabolism
Recombinant Proteins/metabolism/*secretion
Stomach Neoplasms/genetics/metabolism/pathology
Transduction, Genetic
Figure
Reference
-
1. Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int. 2000. 50:767–777.
Article2. Konturek PC, Konturek SJ, Brzozowski T. Gastric cancer and Helicobacter pylori infection. J Physiol Pharmacol. 2006. 57:Suppl 3. 51–65.3. Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993. 90:3539–3543.
Article4. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
Article5. Inaba K, Steinman RM, Pack MW, Aya H, Inaba M, Sudo T, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992. 175:1157–1167.
Article6. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992. 176:1693–1702.
Article7. Greten TF, Jaffee EM. Cancer vaccines. J Clin Oncol. 1999. 17:1047–1060.
Article8. Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 1998. 95:13141–13146.
Article9. Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999. 59:5160–5168.10. Rosenberg SA, Kawakami Y, Robbins PF, Wang R. Identification of the genes encoding cancer antigens: implications for cancer immunotherapy. Adv Cancer Res. 1996. 70:145–177.
Article11. Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996. 183:725–729.
Article12. Huang AY, Bruce AT, Pardoll DM, Levitsky HI. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity. 1996. 4:349–355.
Article13. Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994. 264:961–965.
Article14. Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001. 19:145–156.
Article15. Hege KM, Jooss K, Pardoll D. GM-CSF gene-modified cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006. 25:321–352.16. Sekiguchi M, Sakakibara K, Fujii G. Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med. 1978. 48:61–68.17. Rajagopalan LE, Burkholder JK, Turner J, Culp J, Yang NS, Malter JS. Granulocyte-macrophage colony- stimulating factor mRNA stabilization enhances transgenic expression in normal cells and tissues. Blood. 1995. 86:2551–2558.
Article18. Lange B, Valtieri M, Santoli D, Caracciolo D, Mavilio F, Gemperlein I, et al. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood. 1987. 70:192–199.
Article19. Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991. 115:887–903.
Article20. Jaffee EM, Pardoll DM. Considerations for the clinical development of cytokine gene-transduced tumor cell vaccines. Methods. 1997. 12:143–153.
Article21. Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998. 186:121–130.
Article22. Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, et al. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci U S A. 1994. 91:1858–1862.23. Matozaki T, Sakamoto C, Matsuda K, Suzuki T, Konda Y, Nakano O, et al. Missense mutations and a deletion of the p53 gene in human gastric cancer. Biochem Biophys Res Commun. 1992. 182:215–223.
Article24. Iguchi C, Nio Y, Takeda H, Yamasawa K, Hirahara N, Toga T, et al. Plant polysaccharide PSK: cytostatic effects on growth and invasion; modulating effect on the expression of HLA and adhesion molecules on human gastric and colonic tumor cell surface. Anticancer Res. 2001. 21:1007–1013.25. Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997. 12:473–480.
Article26. Miller AD, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986. 6:2895–2902.
Article27. Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001. 265:11–23.
Article28. Rajagopalan LE, Malter JS. Modulation of granulocyte-macrophage colony-stimulating factor mRNA stability in vitro by the adenosine-uridine binding factor. J Biol Chem. 1994. 269:23882–23888.
Article29. Rajagopalan LE, Malter JS. Turnover and translation of in vitro synthesized messenger RNAs in transfected, normal cells. J Biol Chem. 1996. 271:19871–19876.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor
- Chemopotentiation of Fresh Acute Myelogenous Leukemic Cells by Recombinant Human Granulocyte - Macrophage Colony - Stimulating Factor ( GM-CSF ) and Methotrexate
- Preliminary study of antithymocyte or antilymphocyte globulin, cyclosporine-A and recombinant human granulocyte macrophage colony stimulating factors for patients with aplastic anemia
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- Effect of combination gene therapy with herpes simplex virus thymidine kinase suicidal gene and granulocyte-macrophage colony-stimulating factor gene in murine melanoma model