J Vet Sci.
2010 Dec;11(4):291-297. 10.4142/jvs.2010.11.4.291.
Conditional knockout of brca1/2 and p53 in mouse ovarian surface epithelium: Do they play a role in ovarian carcinogenesis?
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Child and Family Research Institute, University of British Columbia, Vancouver, British Columbia, Canada. kchoi@cbu.ac.kr
- 2Laboratory of Veterinary Biochemistry, College of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- 3Laboratory of Reproductive Biology and Infertility, Cheil General Hospital and Women's Health Center, College of Medicine, Kwandong University, Seoul 100-380, Korea.
- KMID: 1072177
- DOI: http://doi.org/10.4142/jvs.2010.11.4.291
Abstract
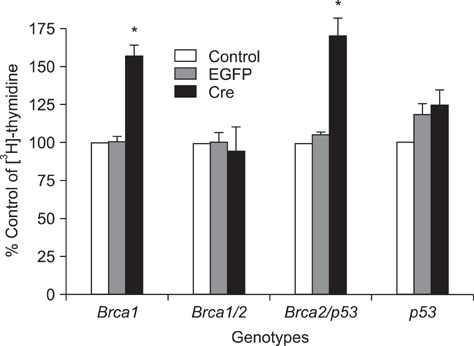
- Alterations of genes are known to be critical for the induction of tumorigenesis, but the mechanism of ovarian carcinogenesis is little understood and remains to be elucidated. In this study, we investigated the roles of brca1, brca2 and p53 genes in the development of ovarian cancer using conditional knockout mice generated by a Cre-loxP recombinant system. Following the application of recombinant adenovirus expressing Cre in vitro, the proliferation of ovarian surface epithelium (OSE) was increased. For instance, a significant increase in cell growth was observed in OSE cells in vitro by conditional knockout isolated from the mice bearing concurrent floxed copies of brca1 and brca2/p53. However, the proliferative effect of the ovarian cells was not observed in concurrent brca1/brca2 or p53 knockout mice in vivo, indicating that we could not observe the direct evidence of the involvement of brca1, brca2, and p53 in ovarian carcinogenesis. Since morphological changes including tumor formation were not observed in mice bearing floxed copies of concurrent brca1/brca2 or p53, the inactivation of brca1/2 or p53 is not sufficient for the induction of tumor formation. Taken together, these results suggest that the deficiency of these genes may not be involved directly in the mechanism of ovarian carcinogenesis.
Keyword
MeSH Terms
-
Animals
BRCA1 Protein/*genetics/metabolism
BRCA2 Protein/*genetics/metabolism
Cell Proliferation
Cell Transformation, Neoplastic/*genetics
Epithelium/*pathology
Extracellular Matrix Proteins/genetics
Female
Gene Silencing
Mice
Mice, Knockout
Ovarian Neoplasms/*genetics
Protein-Lysine 6-Oxidase/genetics
Tumor Cells, Cultured
Tumor Suppressor Protein p53/*genetics/metabolism
Figure
Reference
-
1. Auersperg N, Edelson MI, Mok SC, Johnson SW, Hamilton TC. The biology of ovarian cancer. Semin Oncol. 1998. 25:281–304.2. Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001. 22:255–288.
Article3. Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from brca1 heterozygotes. Cancer. 2000. 89:383–390.
Article4. Brodie SG, Deng CX. brca1-associated tumorigenesis: what have we learned from knockout mice? Trends Genet. 2001. 17:S18–S22.5. Brodie SG, Xu X, Qiao W, Li WM, Cao L, Deng CX. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001. 20:7514–7523.
Article6. Chan KY, Ozçelik H, Cheung AN, Ngan HY, Khoo US. Epigenetic factors controlling the brca1 and brca2 genes in sporadic ovarian cancer. Cancer Res. 2002. 62:4151–4156.7. Chen J, Silver DP, Walpita D, Cantor SB, Gazdar AF, Tomlinson G, Couch FJ, Weber BL, Ashley T, Livingston DM, Scully R. Stable interaction between the products of the brca1 and brca2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998. 2:317–328.
Article8. Choi KC, Kang SK, Tai CJ, Auersperg N, Leung PCK. Estradiol up-regulates antiapoptotic Bcl-2 messenger ribonucleic acid and protein in tumorigenic ovarian surface epithelium cells. Endocrinology. 2001. 142:2351–2360.
Article9. Clark-Knowles KV, Garson K, Jonkers J, Vanderhyden BC. Conditional inactivation of Brca1 in the mouse ovarian surface epithelium results in an increase in preneoplastic changes. Exp Cell Res. 2007. 313:133–145.
Article10. Cressman VL, Backlund DC, Hicks EM, Gowen LC, Godfrey V, Koller BH. Mammary tumor formation in p53- and brca1-deficient mice. Cell Growth Differ. 1999. 10:1–10.11. Deng CX, Brodie SG. Roles of brca1 and its interacting proteins. Bioessays. 2000. 22:728–737.12. Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003. 63:3459–3463.13. Frank TS, Critchfield GC. Identifying and managing hereditary risk of breast and ovarian cancer. Clin Perinatol. 2001. 28:395–406.
Article14. Gowen LC, Johnson BL, Latour AM, Sulik KK, Koller BH. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat Genet. 1996. 12:191–194.
Article15. Gretarsdottir S, Thorlacius S, Valgardsdottir R, Gudlaugsdottir S, Sigurdsson S, Steinarsdottir M, Jonasson JG, Anamthawat-Jonsson K, Eyfjörd JE. brca2 and p53 mutations in primary breast cancer in relation to genetic instability. Cancer Res. 1998. 58:859–862.16. Hedenfalk IA. Gene expression profiling of hereditary and sporadic ovarian cancers reveals unique brca1 and brca2 signatures. J Natl Cancer Inst. 2002. 94:960–961.
Article17. Herbst AL. The epidemiology of ovarian carcinoma and the current status of tumor markers to detect disease. Am J Obstet Gynecol. 1994. 170:1099–1105.
Article18. Hilton JL, Geisler JP, Rathe JA, Hattermann-Zogg MA, DeYoung B, Buller RE. Inactivation of brca1 and brca2 in ovarian cancer. J Natl Cancer Inst. 2002. 94:1396–1406.19. Hosking L, Trowsdale J, Nicolai H, Solomon E, Foulkes W, Stamp G, Signer E, Jeffreys A. A somatic brca1 mutation in an ovarian tumour. Nat Genet. 1995. 9:343–344.20. Koul A, Malander S, Loman N, Pejovic T, Heim S, Willen R, Johannsson O, Olsson H, Ridderheim M, Borg Å. brca1 and brca2 mutations in ovarian cancer: Covariation with specific cytogenetic features. Int J Gynecol Cancer. 2000. 10:289–295.
Article21. Lakhani SR, Manek S, Penault-Llorca F, Flanagan A, Arnout L, Merrett S, McGuffog L, Steele D, Devilee P, Klijn JG, Meijers-Heijboer H, Radice P, Pilotti S, Nevanlinna H, Butzow R, Sobol H, Jacquemier J, Lyonet DS, Neuhausen SL, Weber B, Wagner T, Winqvist R, Bignon YJ, Monti F, Schmitt F, Lenoir G, Seitz S, Hamman U, Pharoah P, Lane G, Ponder B, Bishop DT, Easton DF. Pathology of ovarian cancers in brca1 and brca2 carriers. Clin Cancer Res. 2004. 10:2473–2481.
Article22. Levine DA, Federici MG, Reuter VE, Boyd J. Cell proliferation and apoptosis in BRCA-associated hereditary ovarian cancer. Gynecol Oncol. 2002. 85:431–434.
Article23. Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997. 11:1226–1241.
Article24. Malkin D, Li FP, Strong LC, Fraumeni JF Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA, Friend SH. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990. 250:1233–1238.
Article25. Marmorstein LY, Ouchi T, Aaronson SA. The brca2 gene product functionally interacts with p53 and RAD51. Proc Natl Acad Sci USA. 1998. 95:13869–13874.26. Nicosia SV, Johnson JH. Surface morphology of ovarian mesothelium (surface epithelium) and of other pelvic and extrapelvic mesothelial sites in the rabbit. Int J Gynecol Pathol. 1984. 3:249–260.
Article27. Ramus SJ, Bobrow LG, Pharoah PD, Finnigan DS, Fishman A, Altaras M, Harrington PA, Gayther SA, Ponder BA, Friedman LS. Increased frequency of TP53 mutations in brca1 and brca2 ovarian tumours. Genes Chromosomes Cancer. 1999. 25:91–96.28. Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga JL, Halmos G. Hypothalamic hormones and cancer. Front Neuroendocrinol. 2001. 22:248–291.
Article29. Schlosshauer PW, Cohen CJ, Penault-Llorca F, Miranda CR, Bignon YJ, Dauplat J, Deligdisch L. Prophylactic oophorectomy: a morphologic and immunohistochemical study. Cancer. 2003. 98:2599–2606.30. Scully R, Livingston DM. In search of the tumour-suppressor functions of brca1 and brca2. Nature. 2000. 408:429–432.
Article31. Sekine M, Nagata H, Tsuji S, Hirai Y, Fujimoto S, Hatae M, Kobayashi I, Fujii T, Nagata I, Ushijima K, Obata K, Suzuki M, Yoshinaga M, Umesaki N, Satoh S, Enomoto T, Motoyama S, Tanaka K. Mutational analysis of brca1 and brca2 and clinicopathologic analysis of ovarian cancer in 82 ovarian cancer families: two common founder mutations of brca1 in Japanese population. Clin Cancer Res. 2001. 7:3144–3150.32. Somasundaram K. Breast cancer gene 1 (brca1): role in cell cycle regulation and DNA repair--perhaps through transcription. J Cell Biochem. 2003. 88:1084–1091.
Article33. Suzuki A, de la Pompa JL, Hakem R, Elia A, Yoshida R, Mo R, Nishina H, Chuang T, Wakeham A, Itie A, Koo W, Billia P, Ho A, Fukumoto M, Hui CC, Mak TW. Brca2 is required for embryonic cellular proliferation in the mouse. Genes Dev. 1997. 11:1242–1252.
Article34. Venkitaraman AR. Functions of brca1 and brca2 in the biological response to DNA damage. J Cell Sci. 2001. 114:3591–3598.35. Venkitaraman AR. Cancer susceptibility and the functions of brca1 and brca2. Cell. 2002. 108:171–182.
Article36. Weaver Z, Montagna C, Xu X, Howard T, Gadina M, Brodie SG, Deng CX, Ried T. Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene. 2002. 21:5097–5107.
Article37. Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999. 22:37–43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Mutations in Animal Models: Do Tumor Suppressor Genes, brca1 and brca2, Play a Role in Ovarian Carcinogenesis?
- Expression of BRCA1 Transcripts and Protein in Sporadic Ovarian Cancer
- Expression of Cyclin D1 and CDK4 in DMBA-Induced Rat Ovarian Cancer
- Germline Mutations and polymorphisms of BRCA1 and BRCA2 in Sporadic Ovarian Carcinoma
- A Study on Expression of p53 Protein according to Histologic Types, Degree of Malignancy and Differentiation of the Ovarian Surface Epithelial Tumors