J Vet Sci.
2011 Sep;12(3):273-280. 10.4142/jvs.2011.12.3.273.
Biomarkers for identifying the early phases of osteoarthritis secondary to medial patellar luxation in dogs
- Affiliations
-
- 1Department of Surgery and Obstetrics, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh. alammr74@yahoo.com
- 2Paek Kwang C&S, Sungnam 463-824, Korea.
- 3Department of Surgery, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea.
- KMID: 1067400
- DOI: http://doi.org/10.4142/jvs.2011.12.3.273
Abstract
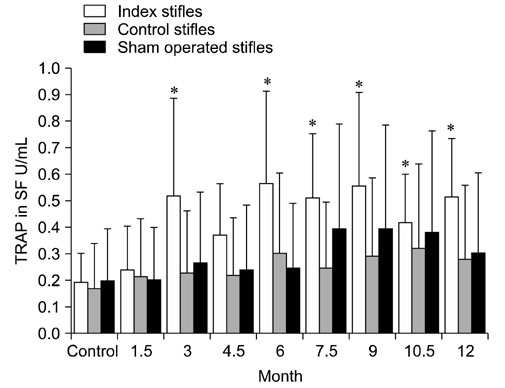
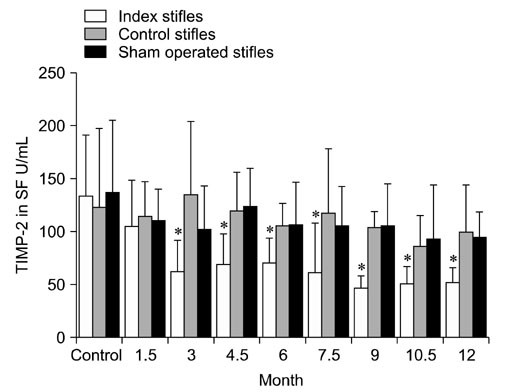
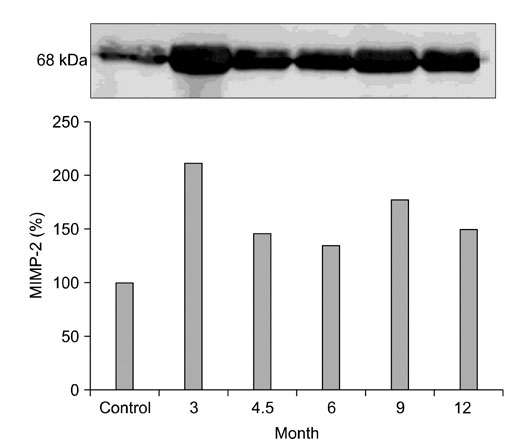
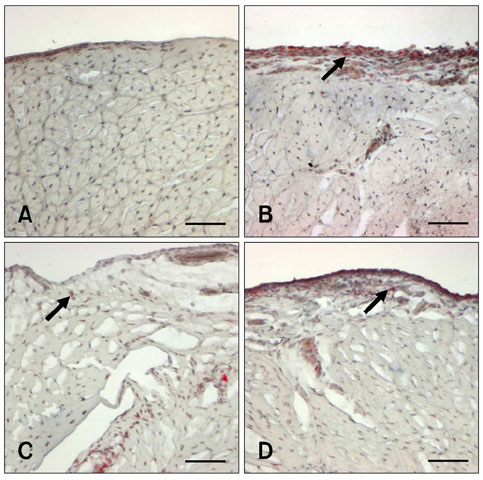
- The levels of tartrate resistant acid phosphatase (TRAP), matrix metalloproteinase-2 (MMP-2), and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in synovial fluid (SF) and serum in cases of canine osteoarthritis (OA) were measured. OA was induced by a surgically-created medial patellar luxation in the left stifle of 24 dogs. SF and blood samples were collected at 1.5- and 3-month intervals, respectively. Every 3 months, one dog was euthanatized to collect tissue samples from both stifles. TRAP levels in SF and serum were measured using a spectrophotometer, and TRAP-positive cells in joint tissues were identified by enzyme histochemistry. MMP-2 and TIMP-2 in SF and serum were detected by Western blotting and ELISA, respectively. TRAP in SF from the stifles and serum was significantly increased (p < 0.05) after 3 months. TIMP-2 in SF and serum was significantly decreased (p < 0.05), whereas MMP-2 in SF was significantly increased (p < 0.05) during the progression of OA. Histochemistry revealed an increased number of TRAP-positive cells in tissues from OA-affected joints. Assays measuring TRAP, MMP-2, and TIMP-2 in SF and serum, and methods that detect increased numbers of TRAP-positive cells in the joint tissues can play an important role in identifying the early phases of degenerative changes in canine joint components.
Keyword
MeSH Terms
-
Acid Phosphatase/analysis/blood
Animals
Arthritis, Experimental/enzymology/etiology/veterinary
Biological Markers/*analysis/*blood
Blotting, Western/veterinary
Dislocations/complications/*veterinary
Dog Diseases/*enzymology/etiology
Dogs
Enzyme-Linked Immunosorbent Assay/veterinary
Female
Isoenzymes/analysis/blood
Male
Matrix Metalloproteinase 2/analysis/blood
Osteoarthritis/enzymology/etiology/*veterinary
Spectrophotometry/veterinary
Stifle/physiopathology
Synovial Fluid/*enzymology
Tissue Inhibitor of Metalloproteinase-2/analysis/blood
Figure
Reference
-
1. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen D, DeCarlo A, Engler JA. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993. 4:197–250.
Article2. Catterall JB, Cawston TE. Assays of matrix metalloproteinases (MMPs) and MMP inhibitors: bioassays and immunoassays applicable to cell culture medium, serum, and synovial fluid. Methods Mol Biol. 2003. 225:353–364.
Article3. Cawston TE. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996. 70:163–182.
Article4. Chevalier X. Is a biological marker for osteoarthritis within reach? Rev Rhum Engl Ed. 1997. 64:562–577.5. Clegg PD, Coughlan AR, Carter SD. Equine TIMP-1 and TIMP-2: Identification, activity and cellular sources. Equine Vet J. 1998. 30:416–423.
Article6. Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989. 84:678–685.
Article7. Fox DB, Cook JL. Synovial fluid markers of osteoarthritis in dogs. J Am Vet Med Assoc. 2001. 219:756–761.
Article8. Halleen JM, Räisänen S, Salo JJ, Reddy SV, Roodman GD, Hentunen TA, Lehenkari PP, Kaija H, Vihko P, Väänänen HK. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J Biol Chem. 1999. 274:22907–22910.
Article9. Hayman AR, Bune AJ, Bradley JR, Rashbass J, Cox TM. Osteoclastic tartrate-resistant acid phosphatase (Acp 5): Its localization to dendritic cells and diverse murine tissues. J Histochem Cytochem. 2000. 48:219–228.
Article10. Hegemann N, Kohn B, Brunnberg L, Schmidt MF. Biomarkers of joint tissue metabolism in canine osteoarthritic and arthritic joint disorders. Osteoarthritis Cartilage. 2002. 10:714–721.
Article11. Janckila AJ, Simons RM, Yam LT. Alternative immunoassay for tartrate-resistant acid phosphatase isoform 5b using the fluorogenic substrate naphthol ASBI-phosphate and heparin. Clin Chim Acta. 2004. 347:157–167.
Article12. Kaija H, Alatalo SL, Halleen JM, Lindquist Y, Schneider G, Vaananen HK, Vihko P. Phosphatase and oxygen radical-generating activities of mammalian purple acid phosphatase are functionally independent. Biochem Biophys Res Commun. 2002. 292:128–132.
Article13. Klimiuk PA, Sierakowski S, Latosiewicz S, Latosiewicz R, Cylwik B, Skowronski J, Chweicko J. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in different histological variants of rheumatoid synovitis. Rheumatology (Oxford). 2002. 41:78–87.
Article14. Lee HB, Alam MR, Seol JW, Kim NS. Tartrate-resistant acid phosphatase, matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in early stages of canine osteoarthritis. Vet Med (Praha). 2008. 53:214–220.
Article15. Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990. 6:121–125.
Article16. Matyas JR, Atley L, Ionescu M, Eyre DR, Poole AR. Analysis of cartilage biomarkers in the early phases of canine experimental osteoarthritis. Arthritis Rheum. 2004. 50:543–552.
Article17. Muir P, Schamberger GM, Manley PA, Hao Z. Localization of cathepsin K and tartrate-resistant acid phosphatase in synovium and cranial cruciate ligament in dogs with cruciate disease. Vet Surg. 2005. 34:239–246.
Article18. Ness MG, Abercromby RH, May C, Turner BM, Carmichael S. A survey of orthopaedic conditions in small animal veterinary practice in Britain. Vet Comp Orthop Traumatol. 1996. 9:43–52.
Article19. Pelletier JP, Mineau F, Faure MP, Martel-Pelletier J. Imbalance between the mechanisms of activation and inhibition of metalloproteases in the early lesions of experimental osteoarthritis. Arthritis Rheum. 1990. 33:1466–1476.
Article20. Roy RG, Wallace LJ, Johnston GR, Wickstrom SL. A retrospective evaluation of stifle osteoarthritis in dogs with bilateral medial patellar luxation and unilateral surgical repair. Vet Surg. 1992. 21:475–479.
Article21. Salinardi BJ, Roush JK, Schermerhorn T, Mitchell KE. Matrix metalloproteinase and tissue inhibitor of metalloproteinase in serum and synovial fluid of osteoarthritic dogs. Vet Comp Orthop Traumatol. 2006. 19:49–55.
Article22. Sibille JC, Doi K, Aisen P. Hydroxyl radical formation and iron-binding proteins. Stimulation by the purple acid phosphatases. J Biol Chem. 1987. 262:59–62.
Article23. Tsuboi H, Matsui Y, Hayashida K, Yamane S, Maeda-Tanimura M, Nampei A, Hashimoto J, Suzuki R, Yoshikawa H, Ochi T. Tartrate resistant acid phosphatase (TRAP) positive cells in rheumatoid synovium may induce the destruction of articular cartilage. Ann Rheum Dis. 2003. 62:196–203.
Article24. Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol. 1999. 30:795–802.
Article25. Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000. 59:455–461.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Patellofemoral contact mechanics after transposition of tibial tuberosity in dogs
- Effects of quadriceps angle on patellofemoral contact pressure
- The clinical implication of sodium-potassium ratios in dogs
- Total Knee Arthroplasty in an Adult with Neglected Patellar Dislocation: A Case Report
- Effects of orthopedic postoperative rehabilitation treatments in dogs