J Vet Sci.
2011 Mar;12(1):75-82. 10.4142/jvs.2011.12.1.75.
In vitro maturation and fertilization of prepubertal and pubertal black Bengal goat oocytes
- Affiliations
-
- 1Department of Surgery and Obstetrics, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh. MohammadBozlur.Rahman@UGent.be
- KMID: 1067340
- DOI: http://doi.org/10.4142/jvs.2011.12.1.75
Abstract
- Oocytes retrieval, in vitro maturation (IVM) and fertilization (IVF) efficiency are inevitable steps towards in vitro production of embryos. In the present study, these parameters were investigated in the ovaries of prepubertal (n = 31) and pubertal (n = 61) black Bengal goats obtained from a slaughterhouse. Nuclear maturation was evaluated upon aspiration and following IVM in TCM-199 (Earle's salt with L-glutamine and sodium bicarbonate) for 27 h at 39degrees C under 5% CO2 in humidified air. The oocytes retrieval and efficiency (mean +/- SD) per prepubertal and pubertal goats were 5.2 +/- 0.6 and 6.8 +/- 0.6, and 77.3 +/- 0.1% and 80.5 +/- 0.6%, respectively. Anaphase I - telophase I stages differed significantly (7.3 +/- 0.8 vs. 2.6 +/- 0.2, p < 0.05) between the two groups of goats. After IVM, the percentages of metaphase II were significantly higher (66.3 vs. 60.3, p < 0.05) in pubertal goats than in their prepubertal counterparts. The percentages of normal in vitro fertilization (IVF) in Fert-Tyrode's albumin lactate pyruvate of pubertal goat oocytes did not differ between Percoll and swim-up sperm separation methods (36.7 +/- 0.9% vs. 32.7 +/- 1.3%, p > 0.05). Furthermore, sperm capacitation by heparin alone or in combination with ionomycin did not lead to a significant increase in the normal fertilization rate (34.8 +/- 1.7 vs. 32.2 +/- 1.5%, respectively) in the oocytes of pubertal goats. In conclusion, the ovaries of pubertal black Bengal goats obtained from the slaughterhouse could be used for in vitro embryo production. However, further optimization of the IVM and IVF techniques are necessary for satisfactory in vitro embryo production.
Keyword
MeSH Terms
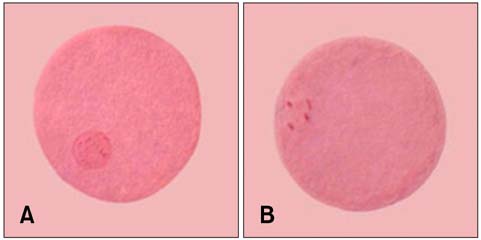
Figure
Reference
-
1. Anguita B, Jimenez-Macedo AR, Izquierdo D, Mogas T, Paramio MT. Effect of oocyte diameter on meiotic competence, embryo development, p34 (cdc2) expression and MPF activity in prepubertal goat oocytes. Theriogenology. 2007. 67:526–536.
Article2. Baldassarre H, Wang B, Kafidi N, Gauthier M, Neveu N, Lapointe J, Sneek L, Leduc M, Duguay F, Zhou JF, Lazaris A, Karatzas CN. Production of transgenic goats by pronuclear microinjection of in vitro produced zygotes derived from oocytes recovered by laparoscopy. Theriogenology. 2003. 59:831–839.
Article3. Bhuiyan MM, Suzuki Y, Watanabe H, Matsuoka K, Fujise Y, Ishikawa H, Ohsumi S, Fukui Y. Attempts at in vitro fertilization and culture of in vitro matured oocytes in sei (Balaenoptera borealis) and Bryde's (B. edeni) whales. Zygote. 2009. 17:19–28.
Article4. Blondin P, Coenen K, Guilbault LA, Sirard MA. In vitro production of bovine embryos: developmental competence is acquired before maturation. Theriogenology. 1997. 47:1061–1075.
Article5. Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2007. CD004507.
Article6. Cesari A, Kaiser GG, Mucci N, Mutto A, Vincenti A, Fornés MW, Alberio RH. Integrated morphophysiological assessment of two methods for sperm selection in bovine embryo production in vitro. Theriogenology. 2006. 66:1185–1193.
Article7. Crozet N, Ahmed-Ali M, Dubos MP. Developmental competence of goat oocytes from follicles of different size categories following maturation, fertilization and culture in vitro. J Reprod Fertil. 1995. 103:293–298.
Article8. Duarte G, Flores JA, Malpaux B, Delgadillo JA. Reproductive seasonality in female goats adapted to a subtropical environment persists independently of food availability. Domest Anim Endocrinol. 2008. 35:362–370.
Article9. Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995. 42:437–442.
Article10. Fulka J Jr, Jung T, Moor RM. The fall of biological maturation promoting factor (MPF) and histone H1 kinase activity during anaphase and telophase in mouse oocytes. Mol Reprod Dev. 1992. 32:378–382.
Article11. Fulka J Jr, Moor RM, Fulka J. Mouse oocyte maturation: meiotic checkpoints. Exp Cell Res. 1995. 219:414–419.
Article12. Galli C, Duchi R, Crotti G, Turini P, Ponderato N, Colleoni S, Lagutina I, Lazzari G. Bovine embryo technologies. Theriogenology. 2003. 59:599–616.
Article13. Gandolfi TA, Gandolfi F. The maternal legacy to the embryo: cytoplasmic components and their effects on early development. Theriogenology. 2001. 55:1255–1276.
Article14. Gibbons A, Bonnet FP, Cueto MI, Catala M, Salamone DF, Gonzalez-Bulnes A. Procedure for maximizing oocyte harvest for in vitro embryo production in small ruminants. Reprod Domest Anim. 2007. 42:423–426.
Article15. Gordon I. Laboratory Production of Cattle Embryos. 1994. Wallingford: CAB International;30–142.16. Han D, Lan GC, Wu YG, Han ZB, Wang HL, Tan JH. Factors affecting the efficiency and reversibility of roscovitine (ROS) block on the meiotic resumption of goat oocytes. Mol Reprod Dev. 2006. 73:238–246.
Article17. Han D, Zhao BT, Liu Y, Li JJ, Wu YG, Lan GC, Tan JH. Interactive effects of low temperature and roscovitine (ROS) on meiotic resumption and developmental potential of goat oocytes. Mol Reprod Dev. 2008. 75:838–846.
Article18. Islam MR, Khandoker MAMY, Afroz S, Rahman MGM, Khan RI. Qualitative and quantitative analysis of goat ovaries, follicles and oocytes in view of in vitro production of embryos. J Zhejiang Univ Sci B. 2007. 8:465–469.
Article19. Izquierdo D, Villamediana P, López-Bejar M, Paramio MT. Effect of in vitro and in vivo culture on embryo development from prepubertal goat IVM-IVF oocytes. Theriogenology. 2002. 57:1431–1441.
Article20. Katska-Ksiazkiewicz L, Opiela J, Ryńska B. Effects of oocyte quality, semen donor and embryo co-culture system on the efficiency of blastocyst production in goats. Theriogenology. 2007. 68:736–744.
Article21. Katska-Ksiazkiewicz L, Ryńska B, Gajda B, Smorag Z. Effect of donor stimulation, frozen semen and heparin treatment on the efficiency of in vitro embryo production in goats. Theriogenology. 2004. 62:576–586.
Article22. Keskintepe L, Darwish GM, Kenimer AT, Brackett BG. Term development of caprine embryos derived from immature oocytes in vitro. Theriogenology. 1994. 42:527–535.
Article23. Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000. 54:741–756.
Article24. King WA, Bousquet D, Greve T, Goff AK. Meiosis in bovine oocytes matured in vitro and in vivo. Acta Vet Scand. 1986. 27:267–279.
Article25. Kruip TAM, Cran DG, van Beneden TH, Dieleman SJ. Structural changes in bovine oocytes during final maturation in vivo. Gamete Res. 1983. 8:29–47.26. Liu RH, Sun QY, Li YH, Jiao LH, Wang WH. Maturation of porcine oocytes after cooling at the germinal vesicle stage. Zygote. 2003. 11:299–305.
Article27. Liu W, Yin Y, Long X, Luo Y, Jiang Y, Zhang W, Du H, Li S, Zheng Y, Li Q, Chen X, Liao B, Xiao G, Wang W, Sun X. Derivation and characterization of human embryonic stem cell lines from poor quality embryos. J Genet Genomics. 2009. 36:229–239.
Article28. Lohuis MM. Potential benefits of bovine embryo-manipulation technologies to genetic improvement programs. Theriogenology. 1995. 43:51–60.
Article29. Lonergan P, Monaghan P, Rizos D, Boland MP, Gordon I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol Reprod Dev. 1994. 37:48–53.
Article30. Long CR, Chase CN, Balise JJ, Duby RT, Robl JM. Effect of sperm removal time, sperm concentration and motility enhancers on fertilization parameters and development of bovine embryos in vitro. Theriogenology. 1993. 39:261.
Article31. Long CR, Damiani P, Pinto-Correia C, MacLean RA, Duby RT, Robl JM. Morphology and subsequent development in culture of bovine oocytes matured in vitro under various conditions of fertilization. J Reprod Fertil. 1994. 102:361–369.
Article32. Luvoni GC. Current progress on assisted reproduction in dogs and cats: in vitro embryo production. Reprod Nutr Dev. 2000. 40:505–512.
Article33. McGaughey RW. Regulation of oocyte maturation. Oxf Rev Reprod Biol. 1983. 5:106–130.34. Mehmood A, Anwar M, Naqvi SM. Motility, acrosome integrity, membrane integrity and oocyte cleavage rate of sperm separated by swim-up or Percoll gradient method from frozen-thawed buffalo semen. Anim Reprod Sci. 2009. 111:141–148.
Article35. Miller GF, Gliedt DW, Rakes JM, Rorie RW. Addition of penicillamine, hypotaurine and epinephrine (PHE) or bovine oviductal epithelial cells (BOEC) alone or in combination to bovine in vitro fertilization medium increases the subsequent embryo cleavage rate. Theriogenology. 1994. 41:689–696.
Article36. Mogas T, Palomo MJ, Izquierdo MD, Paramio MT. Developmental capacity of in vitro matured and fertilized oocytes from prepubertal and adult goats. Theriogenology. 1997. 47:1189–1203.
Article37. Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology. 1997. 48:769–774.
Article38. Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology. 1995. 44:859–869.
Article39. Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, First NL. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology. 1986. 25:591–600.
Article40. Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev. 1992. 31:63–67.
Article41. Pierson J, Wang B, Neveu N, Sneek L, Côté F, Karatzas CN, Baldassarre H. Effects of repetition, interval between treatments and season on the results from laparoscopic ovum pick-up in goats. Reprod Fertil Dev. 2004. 16:795–799.
Article42. Pursel VG, Wall RJ, Rexroad CE, Hammer RE, Brinster RL. A rapid whole-mount staining procedure for nuclei of mammalian embryos. Theriogenology. 1985. 24:687–691.
Article43. Rho GJ, Hahnel AC, Betteridge KJ. Comparisons of oocyte maturation times and of three methods of sperm preparation for their effects on the production of goat embryos in vitro. Theriogenology. 2001. 56:503–516.
Article44. Sirard MA, Parrish JJ, Ware CB, Leibfried-Rutledge ML, First NL. The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod. 1988. 39:546–552.
Article45. Somfai T, Bodó S, Nagy S, Papp AB, Iváncsics J, Baranyai B, Gócza E, Kovács A. Effect of swim up and Percoll treatment on viability and acrosome integrity of frozen-thawed bull spermatozoa. Reprod Domest Anim. 2002. 37:285–290.
Article46. Susko-Parrish JL, Wheeler MB, Ax RL, First NL, Parrish JJ. The effect of penicillamine, hypotaurine, epinephrine and sodium metabisulfite on bovine in vitro fertilization. Theriogenology. 1990. 33:333.
Article47. Talukder AK, Shamsuddin M, Rahman MB, Bari FY, Parrish JJ. Normal and abnormal fertilisation of zebu cattle oocytes in vitro. J Embryo Transf. 2009. 24:89–95.48. Totey SM, Pawshe CH, Singh GP. In vitro maturation and fertilization of buffalo oocytes (Bubalus bubalis): Effects of media, hormones and sera. Theriogenology. 1993. 39:1153–1171.
Article49. Vassena R, Mapletoft RJ, Allodi S, Singh J, Adams GP. Morphology and developmental competence of bovine oocytes relative to follicular status. Theriogenology. 2003. 60:923–932.
Article50. Véliz FG, Poindron P, Malpaux B, Delgadillo JA. Positive correlation between the body weight of anestrous goats and their response to the male effect with sexually active bucks. Reprod Nutr Dev. 2006. 46:657–661.
Article51. Villamediana P, Vidal F, Paramio MT. Cytogenetic analysis of caprine 2- to 4-cell embryos produced in vitro. Zygote. 2001. 9:193–199.
Article52. Wang B, Baldassarre H, Tao T, Gauthier M, Neveu N, Zhou JF, Leduc M, Duguay F, Bilodeau AS, Lazaris A, Keefer C, Karatzas CN. Transgenic goats produced by DNA pronuclear microinjection of in vitro derived zygotes. Mol Reprod Dev. 2002. 63:437–443.
Article53. Watson AJ. Oocyte cytoplasmic maturation: A key mediator of oocyte and embryo developmental competence. J Anim Sci. 2007. 85:13 Suppl. E1–E3.54. Wright RW Jr, Anderson GB, Cupps PT, Drost M, Bradford GE. In vitro culture of embryos from adult and prepuberal ewes. J Anim Sci. 1976. 42:912–917.55. Xu KP, Greve T, Smith S, Hyttel P. Chronological changes of bovine follicular oocyte maturation in vitro. Acta Vet Scand. 1986. 27:505–519.
Article56. Yuan YQ, Van Soom A, Leroy JL, Dewulf J, Van Zeveren A, de Kruif A, Peelman LJ. Apoptosis in cumulus cells, but not in oocytes, may influence bovine embryonic developmental competence. Theriogenology. 2005. 63:2147–2163.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of sperm insemination on the final meiotic maturation of mouse oocytes arrested at metaphase I after in vitro maturation
- Developmental competence of in vitro-matured human oocytes obtained from pregnant and non-pregnant women
- The effect of insemination methods on in vitro maturation outcomes
- Effects of Age on in vitro Maturation and Fertilization of Immature Oocytes from Stimulated Cycles in Human IVF-ET Program
- Morphologic Parameters and in vitro Maturational Competence of Human Immature Oocyte Obtained from Stimulated IVF Cycle