J Vet Sci.
2011 Mar;12(1):35-40. 10.4142/jvs.2011.12.1.35.
Dendrotoxin-kappa suppresses tumor growth induced by human lung adenocarcinoma A549 cells in nude mice
- Affiliations
-
- 1Laboratory of Veterinary Pharmacology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. leeso@snu.ac.kr
- KMID: 1067335
- DOI: http://doi.org/10.4142/jvs.2011.12.1.35
Abstract
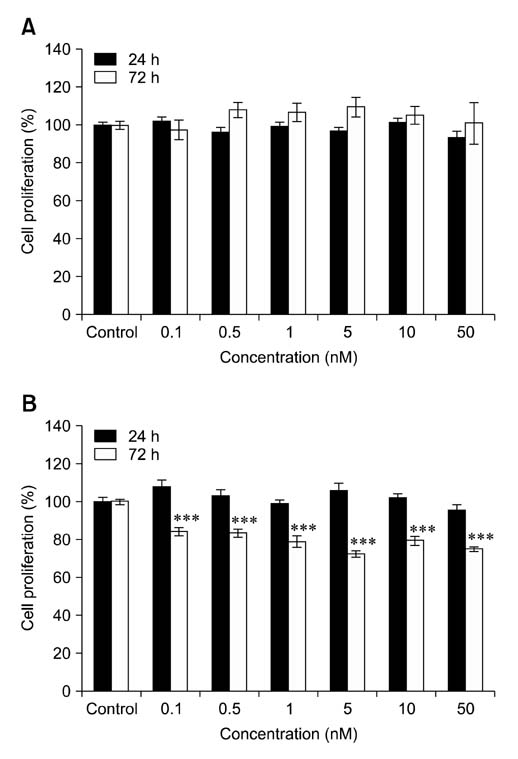
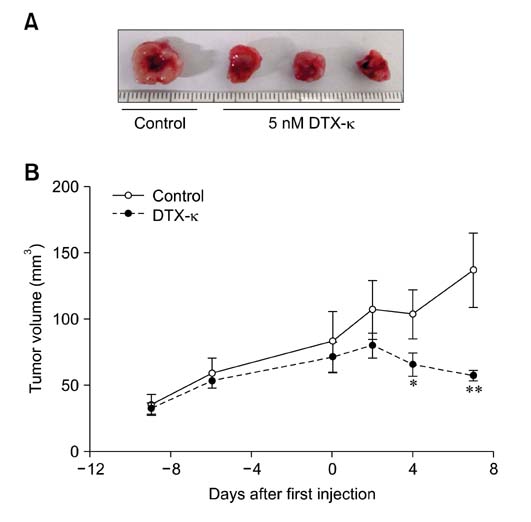
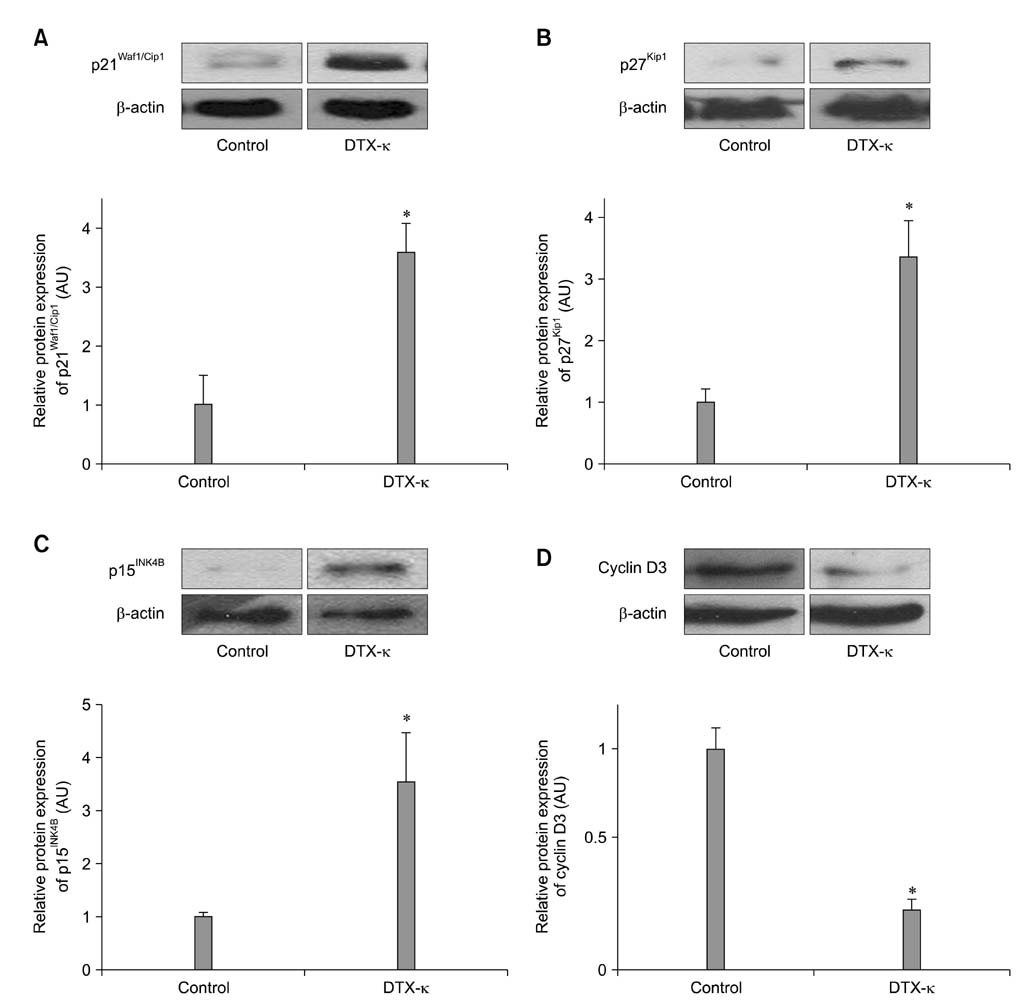
- Voltage-gated K+ (Kv) channels have been considered to be a regulator of membrane potential and neuronal excitability. Recently, accumulated evidence has indicated that several Kv channel subtypes contribute to the control of cell proliferation in various types of cells and are worth noting as potential emerging molecular targets of cancer therapy. In the present study, we investigated the effects of the Kv1.1-specific blocker, dendrotoxin-kappa (DTX-kappa), on tumor formation induced by the human lung adenocarcinoma cell line A549 in a xenograft model. Kv1.1 mRNA and protein was expressed in A549 cells and the blockade of Kv1.1 by DTX-kappa, reduced tumor formation in nude mice. Furthermore, treatment with DTX-kappa significantly increased protein expression of p21Waf1/Cip1, p27Kip1, and p15INK4B and significantly decreased protein expression of cyclin D3 in tumor tissues compared to the control. These results suggest that DTX-kappa has anti-tumor effects in A549 cells through the pathway governing G1-S transition.
MeSH Terms
-
Adenocarcinoma/drug therapy/genetics/pathology
Animals
Cell Line, Tumor
Cell Proliferation/drug effects
Disease Models, Animal
Elapid Venoms/*pharmacology
Elapidae
Humans
Kv1.1 Potassium Channel/*antagonists & inhibitors/deficiency/genetics/metabolism
Lung Neoplasms/*drug therapy/genetics/pathology
Mice
Mice, Nude
Neoplasm Transplantation
Potassium Channel Blockers/*pharmacology
RNA, Messenger/genetics
Transplantation, Heterologous
Figure
Reference
-
1. Abdul M, Santo A, Hoosein N. Activity of potassium channel-blockers in breast cancer. Anticancer Res. 2003. 23:3347–3351.2. Bardou O, Trinh NTN, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009. 296:L145–L155.
Article3. Borowiec AS, Hague F, Harir N, Guénin S, Guerineau F, Gouilleux F, Roudbaraki M, Lassoued K, Ouadid-Ahidouch H. IGF-1 activates hEAG K+ channels through an Akt-dependent signaling pathway in breast cancer cells: role in cell proliferation. J Cell Physiol. 2007. 212:690–701.
Article4. Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G, Jin Y, Wang Y. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int J Cancer. 2009. 125:2281–2287.
Article5. Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, McBain CJ, Gallo V. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci USA. 2002. 99:2350–2355.
Article6. Conti M. Targeting K+ channels for cancer therapy. J Exp Ther Oncol. 2004. 4:161–166.7. Felipe A, Vicente R, Villalonga N, Roura-Ferrer M, Martínez-Mármol R, Solé L, Ferreres JC, Condom E. Potassium channels: new targets in cancer therapy. Cancer Detect Prev. 2006. 30:375–385.
Article8. Gulbins E, Sassi N, Grassmè H, Zoratti M, Szabò I. Role of Kv1.3 mitochondrial potassium channel in apoptotic signalling in lymphocytes. Biochim Biophys Acta. 2010. 1797:1251–1259.
Article9. Harvey AL. Recent studies on dendrotoxins and potassium ion channels. Gen Pharmacol. 1997. 28:7–12.
Article10. Hille B. Ion Channels of Excitable Membranes. 2001. 3rd ed. Sunderland: Sinauer;131–167.11. Huang L, Li B, Li W, Guo H, Zou F. ATP-sensitive potassium channels control glioma cells proliferation by regulating ERK activity. Carcinogenesis. 2009. 30:737–744.
Article12. Jacobs JP, Jones CM, Baille JP. Characteristics of a human diploid cell designated MRC-5. Nature. 1970. 227:168–170.
Article13. Jang SH, Choi C, Hong SG, Yarishkin OV, Bae YM, Kim JG, O'Grady SM, Yoon KA, Kang KS, Ryu PD, Lee SY. Silencing of Kv4.1 potassium channels inhibits cell proliferation of tumorigenic human mammary epithelial cells. Biochem Biophys Res Commun. 2009. 384:180–186.
Article14. Jang SH, Kang KS, Ryu PD, Lee SY. Kv1.3 voltage-gated K+ channel subunit as a potential diagnostic marker and therapeutic target for breast cancer. BMB Rep. 2009. 42:535–539.
Article15. Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999. 39:295–312.
Article16. Lallet-Daher H, Roudbaraki M, Bavencoffe A, Mariot P, Gackière F, Bidaux G, Urbain R, Gosset P, Delcourt P, Fleurisse L, Slomianny C, Dewailly E, Mauroy B, Bonnal JL, Skryma R, Prevarskaya N. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene. 2009. 28:1792–1806.
Article17. Lan M, Shi Y, Han Z, Hao Z, Pan Y, Liu N, Guo C, Hong L, Wang J, Qiao T, Fan D. Expression of delayed rectifier potassium channels and their possible roles in proliferation of human gastric cancer cells. Cancer Biol Ther. 2005. 4:1342–1347.
Article18. Le Guennec JY, Ouadid-Ahidouch H, Soriani O, Besson P, Ahidouch A, Vandier C. Voltage-gated ion channels, new targets in anti-cancer research. Recent Pat Anticancer Drug Discov. 2007. 2:189–202.
Article19. Lee SY, Maniak PJ, Ingbar DH, O'Grady SM. Adult alveolar epithelial cells express multiple subtypes of voltage-gated K+ channels that are located in apical membrane. Am J Physiol Cell Physiol. 2003. 284:C1614–C1624.20. Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995. 17:471–480.
Article21. Ouadid-Ahidouch H, Ahidouch A. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol. 2008. 221:1–6.
Article22. Ouadid-Ahidouch H, Chaussade F, Roudbaraki M, Slomianny C, Dewailly E, Delcourt P, Prevarskaya N. Kv1.1 K+ channels identification in human breast carcinoma cells: involvement in cell proliferation. Biochem Biophys Res Commun. 2000. 278:272–277.
Article23. Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stühmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005. 205:115–124.
Article24. Pardo LA, Sühmer W. Eag1 as a cancer target. Expert Opin Ther Targets. 2008. 12:837–843.
Article25. Rangaraju S, Chi V, Pennington MW, Chandy KG. Kv1.3 potassium channels as a therapeutic target in multiple sclerosis. Expert Opin Ther Targets. 2009. 13:909–924.
Article26. Roura-Ferrer M, Solé L, Martínez-Mármol R, Villalonga N, Felipe A. Skeletal muscle Kv7 (KCNQ) channels in myoblast differentiation and proliferation. Biochem Biophys Res Commun. 2008. 369:1094–1097.
Article27. Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM. Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther. 2008. 7:45–50.
Article28. Shi Y, Ding X, He ZH, Zhou KC, Wang Q, Wang YZ. Critical role of TRPC6 channels in G2 phase transition and the development of human oesophageal cancer. Gut. 2009. 58:1443–1450.
Article29. Smith LA, Reid PF, Wang FC, Parcej DN, Schmidt JJ, Olson MA, Dolly JO. Site-directed mutagenesis of dendrotoxin K reveals amino acids critical for its interaction with neuronal K+ channels. Biochemistry. 1997. 36:7690–7696.
Article30. Spencer RH, Chandy KG, Gutman GA. Immunological identification of the Shaker-related Kv1.3 potassium channel protein in T and B lymphocytes, and detection of related proteins in files and yeast. Biochem Biophys Res Commun. 1993. 191:201–206.
Article31. Stühmer W, Alves F, Hartung F, Zientkowska M, Pardo LA. Potassium channels as tumour markers. FEBS Lett. 2006. 580:2850–2852.
Article32. Villalonga N, Martínez-Mármol R, Roura-Ferrer M, David M, Valenzuela C, Soler C, Felipe A. Cell cycle-dependent expression of Kv1.5 is involved in myoblast proliferation. Biochim Biophys Acta. 2008. 1783:728–736.
Article33. Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, Mansukhani M, Robinson RB, Cordon-Cardo C, Feinmark SJ. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008. 68:1197–1203.
Article34. Wang FC, Bell N, Reid P, Smith LA, McIntosh P, Robertson B, Dolly JO. Identification of residues in dendrotoxin K responsible for its discrimination between neuronal K+ channels containing Kv1.1 and 1.2 alpha subunits. Eur J Biochem. 1999. 263:222–229.
Article35. Wu WK, Li GR, Wong HP, Hui MK, Tai EK, Lam EK, Shin VY, Ye YN, Li P, Yang YH, Luo JC, Cho CH. Involvement of Kv1.1 and Nav1.5 in proliferation of gastric epithelial cells. J Cell Physiol. 2006. 207:437–444.
Article36. Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009. 8:982–1001.
Article37. Yang SL, Cao Q, Zhou KC, Feng YJ, Wang YZ. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009. 28:1320–1328.
Article38. Zeng X, Sikka SC, Huang L, Sun C, Xu C, Jia D, Abdel-Mageed AB, Pottle JE, Taylor JT, Li M. Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation. Prostate Cancer Prostatic Dis. 2009. 13:195–201.
Article39. Zhanping W, Xiaoyu P, Na C, Shenglan W, Bo W. Voltage-gated K+ channels are associated with cell proliferation and cell cycle of ovarian cancer cell. Gynecol Oncol. 2007. 104:455–460.
Article40. Zhao J, Wei XL, Jia YS, Zheng JQ. Silencing of herg gene by shRNA inhibits SH-SY5Y cell growth in vitro and in vivo. Eur J Pharmacol. 2008. 579:50–57.
Article41. Zhou Q, Kwan HY, Chan HC, Jiang JL, Tam SC, Yao X. Blockage of voltage-gated K+ channels inhibits adhesion and proliferation of hepatocarcinoma cells. Int J Mol Med. 2003. 11:261–266.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Incidence Rate and Severity of Orthotopic Lung Cancer in an Animal Model Depends on the Number of A549 Cells and Transplantation Period
- Maspin Suppresses Survival of Lung Cancer Cells through Modulation of Akt Pathway
- Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappa B activation
- Making In Vivo Model To Study About Human Oral Cancer (I)
- Cardamonin Suppresses TGF-beta1-Induced Epithelial Mesenchymal Transition via Restoring Protein Phosphatase 2A Expression