Yonsei Med J.
2011 Nov;52(6):871-878. 10.3349/ymj.2011.52.6.871.
Clinical Implications of Chemokines in Acute and Chronic Hepatitis C Virus Infection
- Affiliations
-
- 1Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon, Korea. ecshin@kaist.ac.kr
- KMID: 1058805
- DOI: http://doi.org/10.3349/ymj.2011.52.6.871
Abstract
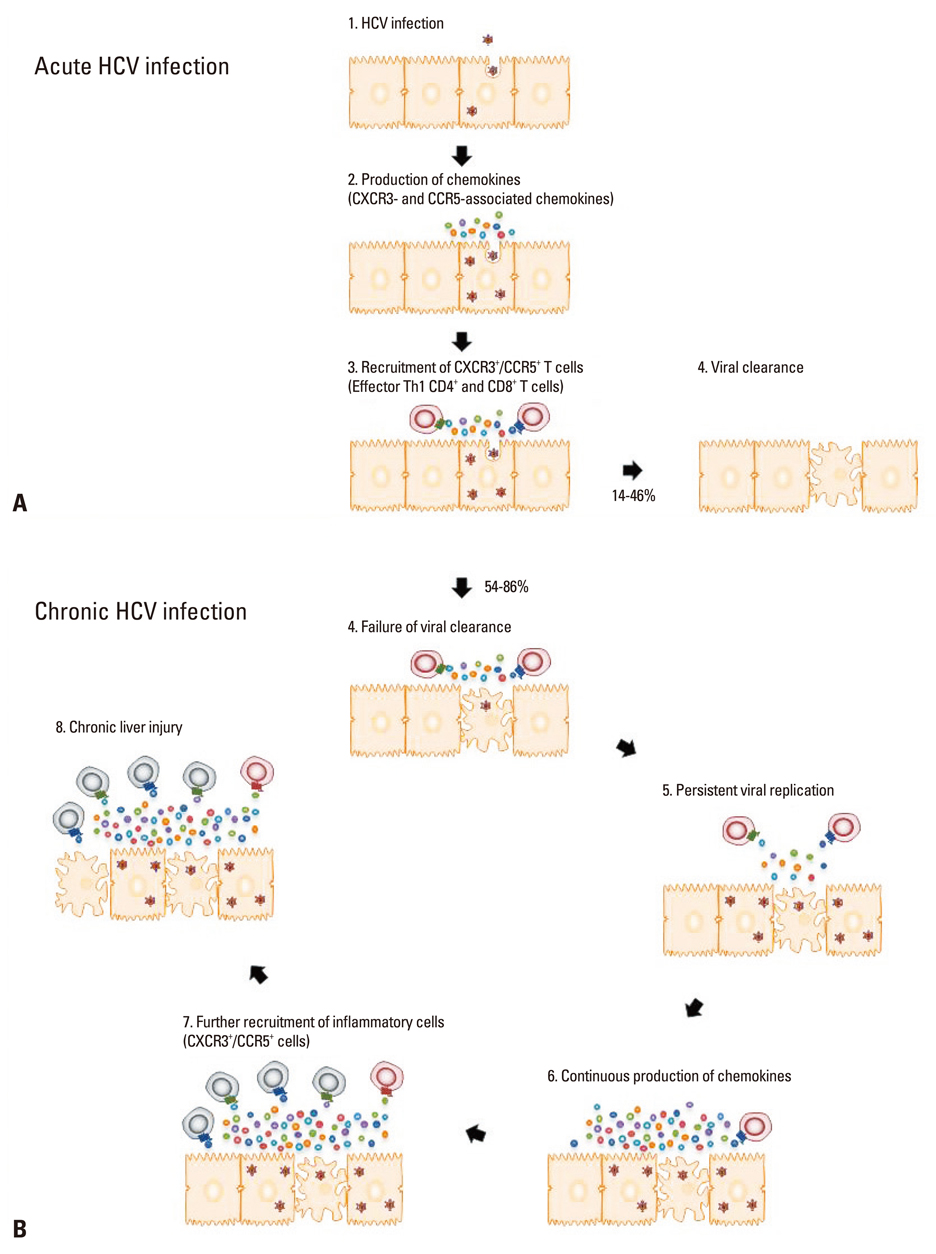
- Hepatitis C virus (HCV), a non-cytopathic positive-stranded RNA virus, is one of the most common causes of chronic liver diseases such as chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. Upon HCV infection, the majority of patients fail to clear the virus and progress to chronic hepatitis C. Chemokines are small chemotactic cytokines that direct the recruitment of immune cells and coordinate immune responses upon viral infection. Chemokine production during acute HCV infection contributes to the recruitment of immune cells with antiviral effector functions and subsequent viral clearance. In chronic HCV infection, however, continuous production of chemokines due to persistent viral replication might result in incessant recruitment of inflammatory cells to the liver, giving rise to persistence of chronic inflammation and liver injury. In this review, we will summarize the roles of chemokines in acute and chronic settings of HCV infection and the clinical relevance of chemokines in the treatment of hepatitis C.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
A Case of Acute Disseminated Encephalomyelitis Associated with Hepatitis C Virus Infection
Jae Eun Sim, Jun-Bum Lee, Yu Na Cho, Sang Hyun Suh, Ja Kyung Kim, Kyung-Yul Lee
Yonsei Med J. 2012;53(4):856-858. doi: 10.3349/ymj.2012.53.4.856.Comparative Analysis of Liver Injury-Associated Cytokines in Acute Hepatitis A and B
So Youn Shin, Sook-Hyang Jeong, Pil Soo Sung, Jino Lee, Hyung Joon Kim, Hyun Woong Lee, Eui-Cheol Shin
Yonsei Med J. 2016;57(3):652-657. doi: 10.3349/ymj.2016.57.3.652.
Reference
-
1. Hepatitis C: global prevalence. Wkly Epidemiol Rec. 1997. 72:341–344.2. Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005. 5:558–567.
Article3. Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000. 20:1–16.
Article4. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002. 36:S35–S46.5. Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004. 24:Suppl 2. 3–8.
Article6. Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000. 191:1499–1512.
Article7. Grüner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000. 181:1528–1536.
Article8. Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, et al. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999. 10:439–449.
Article9. Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002. 99:15661–15668.
Article10. Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000. 95:3032–3043.
Article11. Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000. 18:217–242.
Article12. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000. 12:121–127.13. Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996. 272:60–66.
Article14. Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994. 76:301–314.
Article15. Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003. 38:67–75.
Article16. Heydtmann M, Hardie D, Shields PL, Faint J, Buckley CD, Campbell JJ, et al. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006. 177:729–738.
Article17. Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002. 160:347–355.
Article18. Leroy V, Vigan I, Mosnier JF, Dufeu-Duchesne T, Pernollet M, Zarski JP, et al. Phenotypic and functional characterization of intrahepatic T lymphocytes during chronic hepatitis C. Hepatology. 2003. 38:829–841.
Article19. Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999. 163:6236–6243.20. Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998. 391:344–345.
Article21. Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998. 101:746–754.
Article22. Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004. 34:2907–2918.
Article23. Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol. 2005. 167:887–899.
Article24. Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003. 74:360–369.
Article25. Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999. 104:49–57.
Article26. Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008. 372:321–332.
Article27. Shin EC, Park SH, Demino M, Nascimbeni M, Mihalik K, Major M, et al. Delayed Induction, Not Impaired Recruitment of Specific CD8(+) T Cells, Causes the Late Onset of Acute Hepatitis C. Gastroenterology. 2011. 141:686–695.
Article28. Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, et al. Cross-genotype immunity to hepatitis C virus. J Virol. 2004. 78:1575–1581.
Article29. Major ME, Dahari H, Mihalik K, Puig M, Rice CM, Neumann AU, et al. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology. 2004. 39:1709–1720.
Article30. Zeremski M, Hooker G, Shu MA, Winkelstein E, Brown Q, Des Jarlais DC, et al. Induction of CXCR3- and CCR5-associated chemokines during acute hepatitis C virus infection. J Hepatol. 2011.
Article31. Decalf J, Fernandes S, Longman R, Ahloulay M, Audat F, Lefrerre F, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007. 204:2423–2437.
Article32. Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004. 39:1220–1229.
Article33. Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, et al. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006. 116:3006–3014.
Article34. Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010. 107:7431–7436.
Article35. Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009. 83:3719–3733.
Article36. Wagoner J, Austin M, Green J, Imaizumi T, Casola A, Brasier A, et al. Regulation of CXCL-8 (interleukin-8) induction by double-stranded RNA signaling pathways during hepatitis C virus infection. J Virol. 2007. 81:309–318.
Article37. Volkov Y, Long A, Freeley M, Golden-Mason L, O'Farrelly C, Murphy A, et al. The hepatitis C envelope 2 protein inhibits LFA-1-transduced protein kinase C signaling for T-lymphocyte migration. Gastroenterology. 2006. 130:482–492.
Article38. Sillanpää M, Kaukinen P, Melén K, Julkunen I. Hepatitis C virus proteins interfere with the activation of chemokine gene promoters and downregulate chemokine gene expression. J Gen Virol. 2008. 89:432–443.
Article39. Apolinario A, Diago M, Lo Iacono O, Lorente R, Pérez C, Majano PL, et al. Increased circulating and intrahepatic T-cell-specific chemokines in chronic hepatitis C: relationship with the type of virological response to peginterferon plus ribavirin combination therapy. Aliment Pharmacol Ther. 2004. 19:551–562.
Article40. Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005. 106:1175–1182.
Article41. Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, et al. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997. 158:5536–5544.42. Nishioji K, Okanoue T, Itoh Y, Narumi S, Sakamoto M, Nakamura H, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol. 2001. 123:271–279.
Article43. Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, et al. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006. 194:895–903.
Article44. Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008. 48:1440–1450.
Article45. Apolinario A, Majano PL, Alvarez-Pérez E, Saez A, Lozano C, Vargas J, et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002. 97:2861–2870.
Article46. Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000. 80:415–422.
Article47. Glass WG, Chen BP, Liu MT, Lane TE. Mouse hepatitis virus infection of the central nervous system: chemokine-mediated regulation of host defense and disease. Viral Immunol. 2002. 15:261–272.
Article48. Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, et al. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004. 34:1164–1174.
Article49. Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003. 125:1060–1076.
Article50. Woitas RP, Ahlenstiel G, Iwan A, Rockstroh JK, Brackmann HH, Kupfer B, et al. Frequency of the HIV-protective CC chemokine receptor 5-Delta32/Delta32 genotype is increased in hepatitis C. Gastroenterology. 2002. 122:1721–1728.
Article51. Mangia A, Santoro R, D'agruma L, Andriulli A. HCV chronic infection and CCR5-delta32/delta32. Gastroenterology. 2003. 124:868–869.52. Zhang M, Goedert JJ, O'Brien TR. High frequency of CCR5-delta32 homozygosity in HCV-infected, HIV-1-uninfected hemophiliacs results from resistance to HIV-1. Gastroenterology. 2003. 124:867–868.
Article53. Goulding C, McManus R, Murphy A, MacDonald G, Barrett S, Crowe J, et al. The CCR5-delta32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut. 2005. 54:1157–1161.
Article54. Hellier S, Frodsham AJ, Hennig BJ, Klenerman P, Knapp S, Ramaley P, et al. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003. 38:1468–1476.
Article55. Helbig KJ, George J, Beard MR. A novel I-TAC promoter polymorphic variant is functional in the presence of replicating HCV in vitro. J Clin Virol. 2005. 32:137–143.
Article56. Lagging M, Romero AI, Westin J, Norkrans G, Dhillon AP, Pawlotsky JM, et al. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006. 44:1617–1625.
Article57. Diago M, Castellano G, García-Samaniego J, Pérez C, Fernández I, Romero M, et al. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006. 55:374–379.
Article58. Askarieh G, Alsiö A, Pugnale P, Negro F, Ferrari C, Neumann AU, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010. 51:1523–1530.
Article59. Casrouge A, Decalf J, Ahloulay M, Lababidi C, Mansour H, Vallet-Pichard A, et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest. 2011. 121:308–317.
Article60. Vargas A, Berenguer J, Catalán P, Miralles P, López JC, Cosín J, et al. Association between plasma levels of eotaxin (CCL-11) and treatment response to interferon-alpha and ribavirin in HIV/HCV co-infected patients. J Antimicrob Chemother. 2010. 65:303–306.
Article61. Yoneda S, Umemura T, Joshita S, Ichijo T, Matsumoto A, Yoshizawa K, et al. Serum chemokine levels are associated with the outcome of pegylated interferon and ribavirin therapy in patients with chronic hepatitis C. Hepatol Res. 2011. 41:587–593.
Article62. Akbar H, Idrees M, Butt S, Awan Z, Sabar MF, Rehaman IU, et al. High baseline interleukine-8 level is an Independent risk factor for the achievement of sustained virological response in chronic HCV patients. Infect Genet Evol. 2011. 11:1301–1305.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Viral Hepatitis and Vaccination
- Pre-S Defective Hepatitis B Virus in Patients with Acute and chronic Hepatitis B Virus Infection
- Viral Hepatitis: Focus on Clinical Manifestations of Hepatitis A, B and C
- Acute Pancreatitis Complicating Spontaneous Acute Exacerbation of Chronic Hepatitis B Virus Infection: Case Report and Review of the Literature
- Cytokines in Chronic Hepatitis B and C Virus Infections