Cyclosporine 0.05% Ophthalmic Emulsion for Dry Eye in Korea: A Prospective, Multicenter, Open-Label, Surveillance Study
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. ckjoo@catholic.ac.kr
- 2Department of Ophthalmology and Visual Science, St. Vincent Hospital, The Catholic University of Korea College of Medicine, Suwon, Korea.
- KMID: 1031182
- DOI: http://doi.org/10.3341/kjo.2011.25.6.369
Abstract
- PURPOSE
To assess the effectiveness and tolerability of cyclosporine ophthalmic emulsion (CsA) 0.05% in patients with moderate to severe dry eye disease in Korea.
METHODS
This was a prospective, multicenter, open-label, surveillance study of 392 Korean patients with moderate to severe dry eye disease who were treated with CsA 0.05% for three months. An assessment of effectiveness was performed at baseline, and after 1, 2, and 3 months. The primary effectiveness outcomes were changes in ocular symptoms and Schirmer score. The secondary effectiveness outcomes were a change in conjunctival staining, use of artificial tears, global evaluation of treatment, and patient satisfaction. The primary safety outcome was the incidence and nature of adverse events.
RESULTS
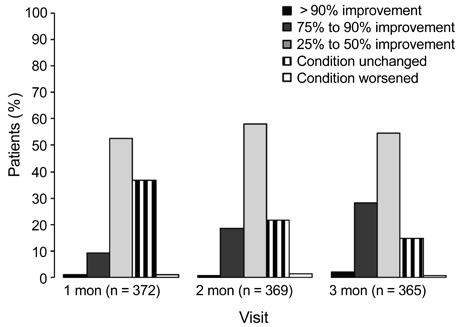
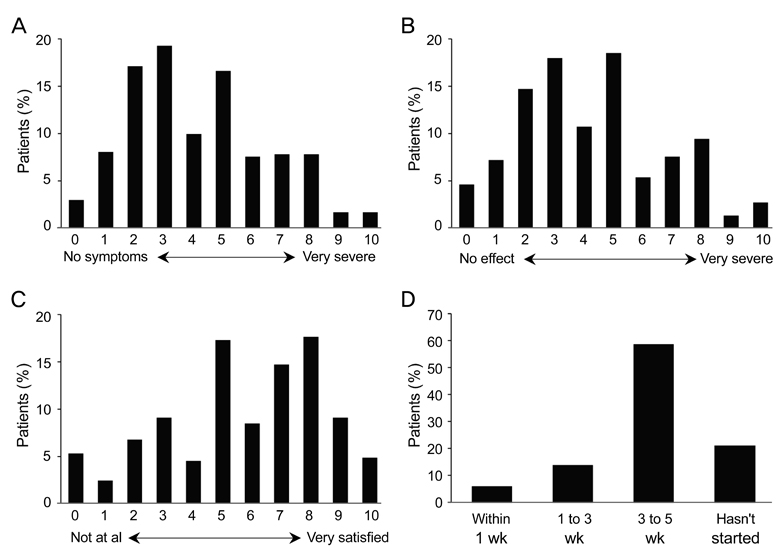
A total of 362 patients completed the study. After three months, all ocular symptom scores were significantly reduced compared to the baseline values, while the Schirmer scores were significantly increased relative to baseline (p < 0.0001). After three months, there were significant reductions from baseline in conjunctival staining (p < 0.01) and use of artificial tears (p < 0.0001). According to clinicians' global evaluations, most patients (>50%) experienced at least a 25% to 50% improvement in symptoms from baseline at each follow-up visit. The majority of patients (72.0%) were satisfied with the treatment results, and 57.2% reported having no or mild symptoms after treatment. The most common adverse events were ocular pain (11.0%).
CONCLUSIONS
Our findings indicate that CsA 0.05% is an effective and tolerable treatment for dry eye disease in Korean clinical practice.
MeSH Terms
-
Cyclosporine/*administration & dosage/adverse effects
Dry Eye Syndromes/*drug therapy/epidemiology
Emulsions
Female
Follow-Up Studies
Humans
Immunosuppressive Agents/*administration & dosage/adverse effects
Male
Middle Aged
Population Surveillance
Prospective Studies
Republic of Korea/epidemiology
Treatment Outcome
Figure
Cited by 3 articles
-
Effects of Cyclosporine 0.05% Ophthalmic Emulsion to Improve Reduction of Tear Production after Cataract Surgery
Ae Ri Yoo, Hyung Bin Hwang, Hyun Kyung Kim, Sung Kun Chung
J Korean Ophthalmol Soc. 2013;54(7):1013-1018. doi: 10.3341/jkos.2013.54.7.1013.Effect of Cyclosporin A on Tear Film and Corneal Aberration after Cataract Surgery
Jei Hun Jeon, Hong Seok Kim, Ji Won Jung, Sang Chul Yoon, Kyoung Yul Seo, Hyung Keun Lee, Eung Kweon Kim, Tae Im Kim
J Korean Ophthalmol Soc. 2014;55(7):978-983. doi: 10.3341/jkos.2014.55.7.978.Comparisons for Evaluation of Efficacy and Safety of Cyclosporin A 0.05% Ophthalmic Emulsion Treatment Groups
Soonwon Yang, Yong-Soo Byun, Chang Rae Rho, Su Young Kim, Yang Kyung Cho, Eun Chul Kim, Sung Kun Chung, Choun-Ki Joo
J Korean Ophthalmol Soc. 2016;57(12):1849-1856. doi: 10.3341/jkos.2016.57.12.1849.
Reference
-
1. O'Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004. 4:314–319.2. Hikichi T, Yoshida A, Fukui Y, et al. Prevalence of dry eye in Japanese eye centers. Graefes Arch Clin Exp Ophthalmol. 1995. 233:555–558.3. Jamaliah R, Fathilah J. Prevalence of dry eye in University Malaya Medical Centre. Med J Malaysia. 2002. 57:390–397.4. Lekhanont K, Rojanaporn D, Chuck RS, Vongthongsri A. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006. 25:1162–1167.5. Shimmura S, Shimazaki J, Tsubota K. Results of a population-based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea. 1999. 18:408–411.6. Lee AJ, Lee J, Saw SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002. 86:1347–1351.7. Lin PY, Cheng CY, Hsu WM, et al. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2005. 46:1593–1598.8. Lu P, Chen X, Liu X, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008. 27:545–551.9. Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond). 2009. 23:688–693.10. Doughty MJ, Fonn D, Richter D, et al. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci. 1997. 74:624–631.11. McCarty CA, Bansal AK, Livingston PM, et al. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998. 105:1114–1119.12. Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000. 118:1264–1268.13. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007. 5:93–107.14. Reddy P, Grad O, Rajagopalan K. The economic burden of dry eye: a conceptual framework and preliminary assessment. Cornea. 2004. 23:751–761.15. Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008. 14:3 Suppl. S102–S106.16. Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007. 143:409–415.17. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007. 5:75–92.18. Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt. 2009. 29:573–583.19. Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009. 3:405–412.20. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007. 5:163–178.21. Sall K, Stevenson OD, Mundorf TK, Reis BL. CsA Phase 3 Study Group. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000. 107:631–639.22. Stevenson D, Tauber J, Reis BL. The Cyclosporin A Phase 2 Study Group. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Ophthalmology. 2000. 107:967–974.23. Perry HD, Solomon R, Donnenfeld ED, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008. 126:1046–1050.24. Moon JW, Lee HJ, Shin KC, et al. Short term effects of topical cyclosporine and viscoelastic on the ocular surfaces in patients with dry eye. Korean J Ophthalmol. 2007. 21:189–194.25. Kim EC, Choi JS, Joo CK. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. 2009. 147:206–213.26. Byun YS, Jeon EJ, Chung SK. Clinical effect of cyclosporine 0.05% eye drops in dry eye syndrome patients. J Korean Ophthalmol Soc. 2008. 49:1583–1588.27. Lee JS, Yoon TJ, Kim KH. Cinical effect of Restasis eye drops in mild dry eye syndrome. J Korean Ophthalmol Soc. 2009. 50:1489–1494.28. Hanson J, Fikertscher R, Roseburg B. Schirmer test of lacrimation. Its clinical importance. Arch Otolaryngol. 1975. 101:293–295.29. Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin. 2005. 21:1057–1063.30. Trattler W, Katsev D, Kerney D. Self-reported compliance with topical cyclosporine emulsion 0.05% and onset of the effects of increased tear production as assessed through patient surveys. Clin Ther. 2006. 28:1848–1856.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Affecting Compliance With 0.05% Cyclosporine Emulsion in Patients With Dry Eye Syndrome
- Study of Utilization Pattern and Compliance with Topical 0.05% Cyclosporine Emulsion in Korean Dry Eye Patients
- Long-term Evaluation After Topical Cyclosporine Treatment in Dry Eye Patients With Graft-Versus-Host Disease
- The Effect of Topical Cyclosporine 0.05% on Dry Eye after Cataract Surgery
- The Efficacies and Safeties of a 0.05% Cyclosporine Nanoemulsion and a 0.1% Cyclosporine Cationic Emulsion