Ann Lab Med.
2015 May;35(3):314-320. 10.3343/alm.2015.35.3.214.
Investigation of Serum Angiotensin II Type 1 Receptor Antibodies at the Time of Renal Allograft Rejection
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. ejoh@catholic.ac.kr
- 2Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2363203
- DOI: http://doi.org/10.3343/alm.2015.35.3.214
Abstract
- BACKGROUND
Angiotensin II type 1 receptor (AT1R) is responsible for cardiovascular effects mediated by angiotensin II. This study aimed to investigate the impact of antibodies directed against AT1R (anti-AT1R) in renal allograft rejection.
METHODS
We evaluated 53 patients who had biopsy-proven rejection including antibody-mediated rejection (AMR) (N=22), T-cell-mediated rejection (TCMR) (N=29), and mixed AMR and TCMR (N=2). Donor specific HLA antibodies (DSA) and anti-AT1Rs were simultaneously determined.
RESULTS
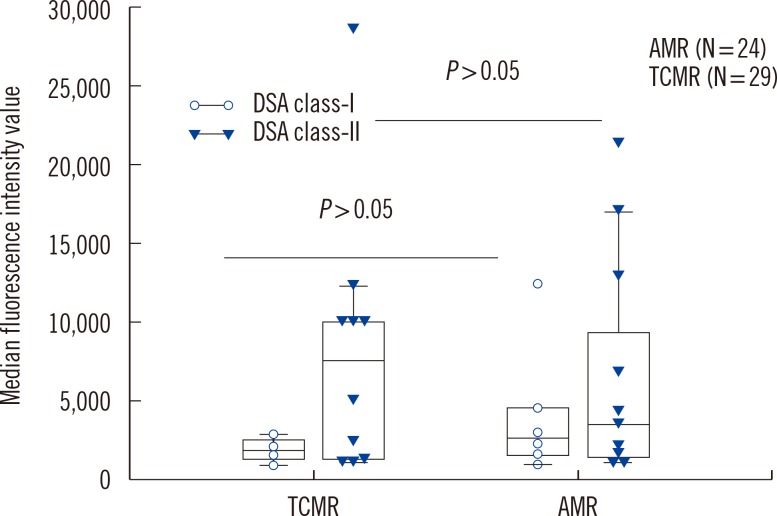
Anti-AT1Rs were detected in 9.4% (5/53) of rejection patients (one with acute AMR, two with chronic active AMR, one with acute TCMR, and one with mixed acute AMR & TCMR). HLA antibodies and DSA were detected in 75.5% (40/53) and 49.1% (26/53) of patients, respectively. There was no significant difference in transplant characteristics between anti-AT1R(+) and anti-AT1R(-) patients except for the association of HLA class-I DSA(+) and anti-AT1R(+). Four of five anti-AT1R(+) patients had DSA and were also found to have AMR. A single anti-AT1R(+)/DSA(-) patient developed acute TCMR. Detection rates of DSA, HLA antibodies, or anti-AT1R were not different between AMR and TCMR. However, DSA(+)/anti-AT1R(+) was more frequently found in AMR than in TCMR (P=0.036). Patients with anti-AT1R showed a greater tendency to develop high-grade rejection as Banff IIA/IIB or AMR.
CONCLUSIONS
The presence of anti-AT1R was significantly associated with HLA class-I DSA in renal allograft rejection patients. Both anti-AT1R and DSA positivity was associated with AMR in patients with renal allograft rejection.
MeSH Terms
Figure
Reference
-
1. Giral M, Foucher Y, Dufay A, Van Huyen JP, Renaudin K, Moreau A, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013; 13:2567–2576. PMID: 23919486.
Article2. Cox ST, Stephens HA, Fernando R, Karasu A, Harber M, Howie AJ, et al. Major histocompatibility complex class I-related chain A allele mismatching, antibodies, and rejection in renal transplantation. Hum Immunol. 2011; 72:827–834. PMID: 21664940.
Article3. Banasik M, Boratyńska M, Kościelska-Kasprzak K, Kamińska D, Bartoszek D, Zabińska M, et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl Int. 2014; 27:1029–1038. PMID: 24909812.
Article4. Reinsmoen NL. Role of angiotensin II type 1 receptor-activating antibodies in solid organ transplantation. Hum Immunol. 2013; 74:1474–1477. PMID: 23831255.
Article5. Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005; 352:558–569. PMID: 15703421.
Article6. Reinsmoen NL, Lai CH, Heidecke H, Haas M, Cao K, Ong G, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010; 90:1473–1477. PMID: 21030904.
Article7. Taniguchi M, Rebellato LM, Cai J, Hopfield J, Briley KP, Haisch CE, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013; 13:2577–2589. PMID: 23941128.
Article8. Kelsch R, Everding AS, Kuwertz-Bröking E, Brand E, Spriewald BM, Sibrowski W, et al. Accelerated kidney transplant rejection and hypertensive encephalopathy in a pediatric patient associated with antibodies against angiotensin type 1 receptor and HLA class II. Transplantation. 2011; 92:e57–e59. PMID: 22067217.
Article9. Chung BH, Choi BS, Oh EJ, Park CW, Kim JI, Moon IS, et al. Clinical impact of the baseline donor-specific anti-human leukocyte antigen antibody measured by Luminex single antigen assay in living donor kidney transplant recipients after desensitization therapy. Transpl Int. 2014; 27:49–59. PMID: 24118413.
Article10. Kim Y, Park KH, Chung BH, Choi BS, Yang CW, Kim JI, et al. Pretransplant IFN-γ ELISPOT assay as a potential screening test to select immunosuppression protocols for patients receiving basiliximab induction therapy. Transl Res. 2012; 160:230–236. PMID: 22683414.
Article11. Jang JY, Kim YJ, Kim Y, Park YJ, Han K, Oh EJ. Application of calculated panel reactive antibody using HLA frequencies in Koreans. Ann Lab Med. 2012; 32:66–72. PMID: 22259781.
Article12. Chung HY, Yoon JA, Han BY, Song EY, Park MH. Allelic and haplotypic diversity of HLA-A, -B, -C, and -DRB1 genes in Koreans defined by high-resolution DNA typing. Korean J Lab Med. 2010; 30:685–696. PMID: 21157157.
Article13. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–760. PMID: 18294345.
Article14. Amico P, Hönger G, Bielmann D, Lutz D, Garzoni D, Steiger J, et al. Incidence and prediction of early antibody-mediated rejection due to non-human leukocyte antigen-antibodies. Transplantation. 2008; 85:1557–1563. PMID: 18551059.
Article15. Reinsmoen NL, Lai CH, Mirocha J, Cao K, Ong G, Naim M, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014; 97:595–601. PMID: 24162250.
Article16. Martin L, Guignier F, Mousson C, Rageot D, Justrabo E, Rifle G. Detection of donor-specific anti-HLA antibodies with flow cytometryin eluates and sera from renal transplant recipients with chronic allograft nephropathy. Transplantation. 2003; 76:395–400. PMID: 12883199.17. Scornik JC, Guerra G, Schold JD, Srinivas TR, Dragun D, Meier-Kriesche HU. Value of posttransplant antibody tests in the evaluation of patients with renal graft dysfunction. Am J Transplant. 2007; 7:1808–1814. PMID: 17524074.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Investigation of Serum Angiotensin II Type 1 Receptor Antibodies at the Time of Renal Allograft Rejection
- Angiotensin II type 1 receptor antibodies in kidney transplantation
- Clinical Impact of Pre-transplant Antibodies Against Angiotensin II Type I Receptor and Major Histocompatibility Complex Class I-Related Chain A in Kidney Transplant Patients
- Effects of Angiotensin II on ZO-1 in Glomerular Epithelial Cells
- Angiotensin converting enqyme inhibitor and angiotensin II AT1 receptor antagonist in progressive renal disease