Intest Res.
2024 Apr;22(2):152-161. 10.5217/ir.2023.00095.
Assessing quality of magnetic resonance enterography and its impact on disease assessment of ileal Crohn’s disease
- Affiliations
-
- 1Department of Gastroenterology, Box Hill Hospital, Box Hill, Australia
- 2Department of Gastroenterology, Northern Hospital, Epping, Australia
- 3Eastern Health Clinical School, Monash University, Box Hill, Australia
- 4Department of Gastroenterology, The Royal Melbourne Hospital, Parkville, Australia
- 5Department of Radiology, Box Hill Hospital, Box Hill, Australia
- KMID: 2554659
- DOI: http://doi.org/10.5217/ir.2023.00095
Abstract
- Background/Aims
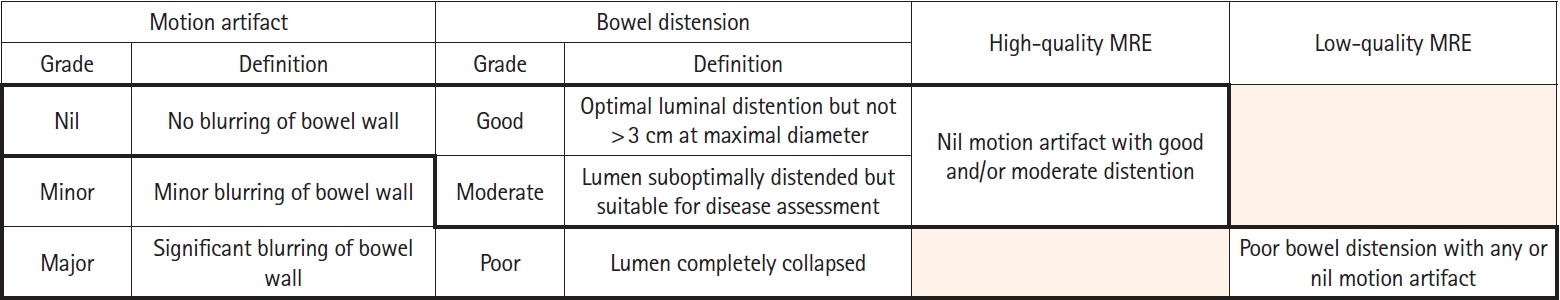
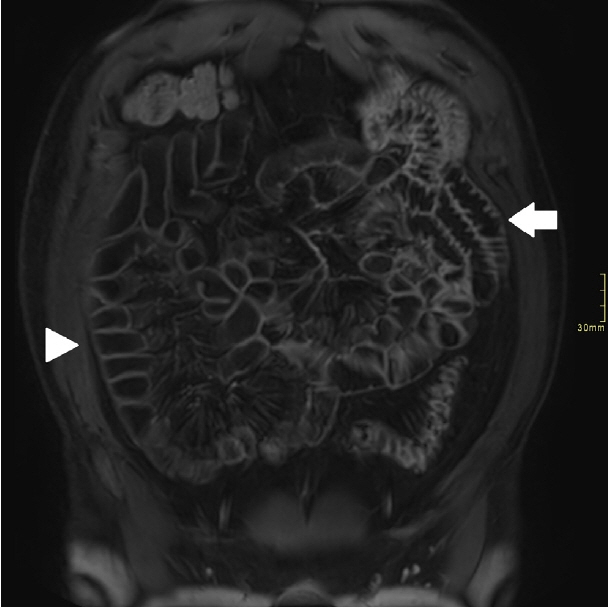
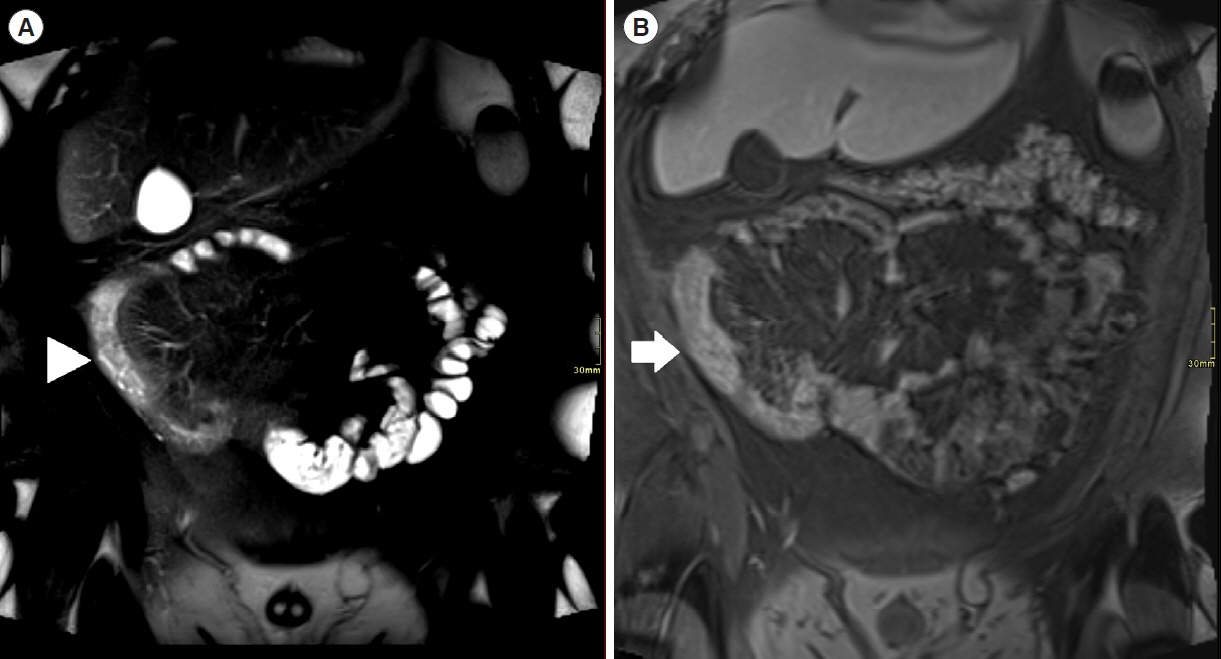
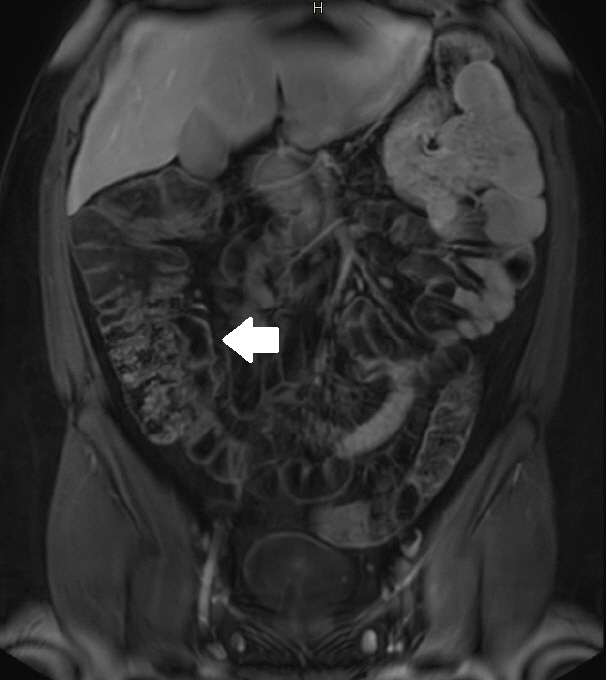
Assessment of quality of magnetic resonance enterography (MRE) in small bowel Crohn’s disease (CD) activity evaluation has received little attention. We assessed the impact of bowel distention and motion artifact on MRE activity indices in ileal CD.
Methods
A cohort of patients who underwent contemporaneous MRE and colonoscopy for ileal CD assessment between 2014 and 2021 at 2 centers were audited. An abdominal radiologist blinded to clinical data reviewed each MRE, graded bowel distention and motion artifact upon a pre-specified 3-point scale and calculated the original magnetic resonance index of activity (MaRIA) and simplified MaRIA (sMaRIA), London index and CD MRE index (CDMI). Ileal endoscopic activity was graded via the Simplified Endoscopy Score for CD (SES-CD). The performance of MRE indices in discriminating active disease (SES-CD ≥3) stratified by MRE quality was measured by receiver operator characteristic analyses.
Results
One hundred and thirty-seven patients had MRE and colonoscopy within a median of 16 days (range, 0–30 days) with 63 (46%) exhibiting active disease (SES-CD ≥3). Forty-four MREs (32%) were deemed low quality due to motion artifact and/or moderate to poor distention. Low-quality MREs demonstrated reduced discriminative performance between ileal SES-CD ≥3 and MRE indices (MaRIA 0.838 vs. 0.634, sMaRIA 0.834 vs. 0.527, CDMI 0.850 vs. 0.595, London 0.748 vs. 0.511, P<0.05 for all). Individually the presence of any motion artifact markedly impacted the discriminative performance (e.g., sMaRIA area under the curve 0.544 vs. 0.814, P<0.05).
Conclusions
Image quality parameters can significantly impact MRE disease activity interpretation. Quality metrics should be reported, enabling cautious interpretation in lower-quality studies.
Keyword
Figure
Cited by 1 articles
-
Achieving high-quality magnetic resonance enterography is critical for assessing Crohn’s disease activity
Kyoung Doo Song
Intest Res. 2024;22(2):117-118. doi: 10.5217/ir.2024.0043.
Reference
-
1. Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers. 2020; 6:22.
Article2. Cushing K, Higgins PD. Management of Crohn disease: a review. JAMA. 2021; 325:69–80.3. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778.
Article4. Berg DR, Colombel JF, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2019; 25:1896–1905.
Article5. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article6. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.
Article7. ASGE Standards of Practice Committee, Fisher DA, Maple JT, et al. Complications of colonoscopy. Gastrointest Endosc. 2011; 74:745–752.
Article8. Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn’s disease. Gastroenterology. 2019; 157:432–439.
Article9. Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009; 58:1113–1120.
Article10. Buisson A, Joubert A, Montoriol PF, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther. 2013; 37:537–545.
Article11. Qiu Y, Mao R, Chen BL, et al. Systematic review with meta-analysis: magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment Pharmacol Ther. 2014; 40:134–146.
Article12. Taylor SA, Avni F, Cronin CG, et al. The first joint ESGAR/ESPR consensus statement on the technical performance of crosssectional small bowel and colonic imaging. Eur Radiol. 2017; 27:2570–2582.
Article13. Grand DJ, Guglielmo FF, Al-Hawary MM. MR enterography in Crohn’s disease: current consensus on optimal imaging technique and future advances from the SAR Crohn’s Disease-Focused Panel. Abdom Imaging. 2015; 40:953–964.
Article14. Griffin N, Grant LA, Anderson S, Irving P, Sanderson J. Small bowel MR enterography: problem solving in Crohn’s disease. Insights Imaging. 2012; 3:251–263.
Article15. Herraiz Hidalgo L, Alvarez Moreno E, Carrascoso Arranz J, Cano Alonso R, Martínez de Vega Fernández V. Magnetic resonance enterography: review of the technique for the study of Crohn’s disease. Radiologia. 2011; 53:421–433.
Article16. Bohra A, Vasudevan A, Kutaiba N, Van Langenberg DR. Challenges and strategies to optimising the quality of small bowel magnetic resonance imaging in Crohn’s disease. Diagnostics (Basel). 2022; 12:2533.
Article17. Saini S, Colak E, Anthwal S, Vlachou PA, Raikhlin A, Kirpalani A. Comparison of 3% sorbitol vs psyllium fibre as oral contrast agents in MR enterography. Br J Radiol. 2014; 87:20140100.18. Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012; 81:2080–2088.
Article19. Rozendorn N, Amitai MM, Eliakim RA, Kopylov U, Klang E. A review of magnetic resonance enterography-based indices for quantification of Crohn’s disease inflammation. Therap Adv Gastroenterol. 2018; 11:1756284818765956.
Article20. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845.
Article21. Pepe M, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. Stata J. 2009; 9:1.
Article22. Ajaj W, Lauenstein TC, Langhorst J, et al. Small bowel hydroMR imaging for optimized ileocecal distension in Crohn’s disease: should an additional rectal enema filling be performed? J Magn Reson Imaging. 2005; 22:92–100.
Article23. Rao A, Sitheeque F, Gustafson S, Lu M, Prior M. MR enterography: impact on image quality between single- versus split-dose Buscopan. J Med Imaging Radiat Oncol. 2020; 64:331–337.
Article24. Froehlich JM, Daenzer M, von Weymarn C, Erturk SM, Zollikofer CL, Patak MA. Aperistaltic effect of hyoscine N-butylbromide versus glucagon on the small bowel assessed by magnetic resonance imaging. Eur Radiol. 2009; 19:1387–1393.
Article25. Vu KN, Haldipur AG, Roh AT, Lindholm P, Loening AM. Comparison of end-expiration versus end-inspiration breath-holds with respect to respiratory motion artifacts on T1-weighted abdominal MRI. AJR Am J Roentgenol. 2019; 212:1024–1029.
Article26. Koplay M, Guneyli S, Cebeci H, et al. Magnetic resonance enterography with oral mannitol solution: diagnostic efficacy and image quality in Crohn disease. Diagn Interv Imaging. 2017; 98:893–899.
Article27. Grand DJ, Beland MD, Machan JT, Mayo-Smith WW. Detection of Crohn’s disease: comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. Eur J Radiol. 2012; 81:1735–1741.
Article28. Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009; 193:113–121.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Computed Tomography Enterography/Magnetic Resonance Enterography: Is It in Prime Time?
- Pediatric Magnetic Resonance Enterography: Focused on Crohn's Disease
- Achieving high-quality magnetic resonance enterography is critical for assessing Crohn’s disease activity
- Computed Tomography Enterography and Magnetic Resonance Enterography in the Diagnosis of Crohn's Disease
- A Look into the Small Bowel in Crohn's Disease