Endocrinol Metab.
2024 Feb;39(1):83-89. 10.3803/EnM.2024.101.
The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin
- Affiliations
-
- 1Division of Endocrinology, Department of Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands
- KMID: 2552798
- DOI: http://doi.org/10.3803/EnM.2024.101
Abstract
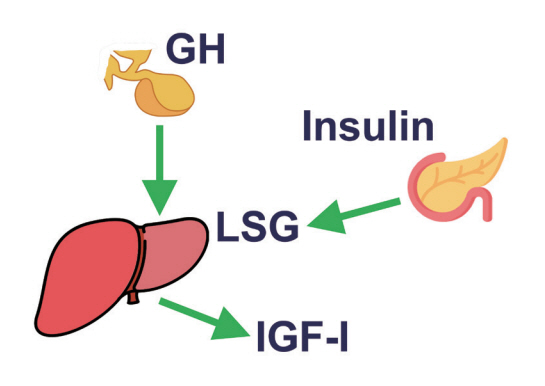
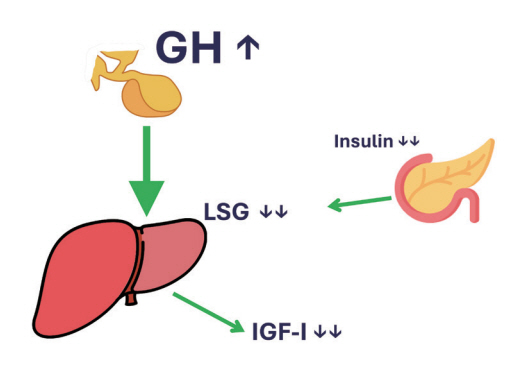
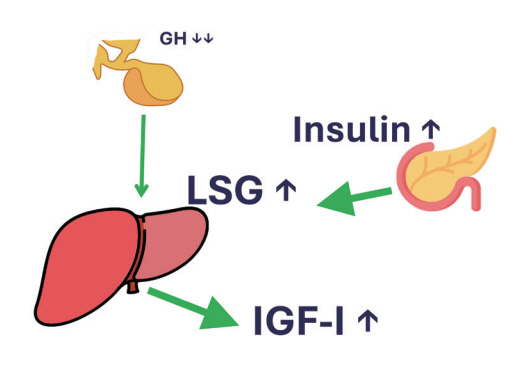
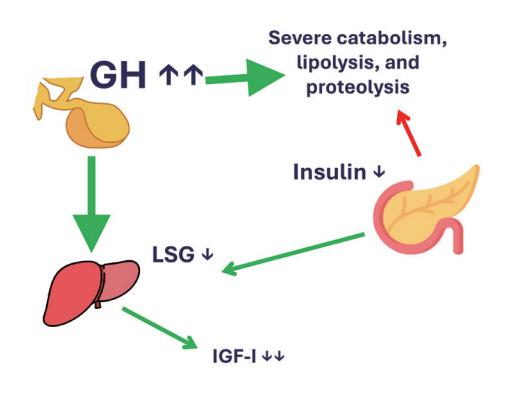
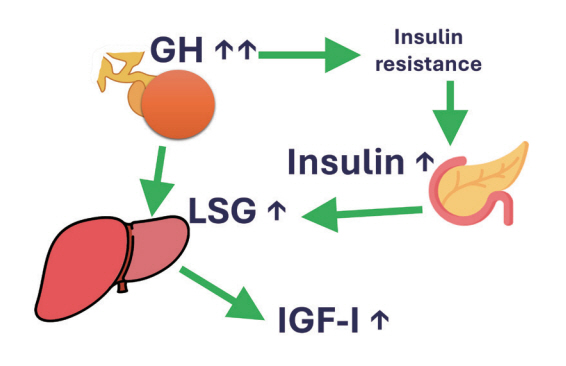
- This review intends to provide the reader with a practical overview of several (patho)physiological conditions in which knowledge of the interplay between growth hormone (GH), insulin-like growth factor-1 (IGF-1), and insulin is important. This might help treating physicians in making the right decisions on how to intervene and improve metabolism for the benefit of patients, and to understand why and how metabolism responds in their specific cases. We will specifically address the interplay between GH, IGF-1, and insulin in type 1 and 2 diabetes mellitus, liver cirrhosis, and acromegaly as examples in which this knowledge is truly necessary.
Keyword
Figure
Reference
-
1. Redondo MJ, Morgan NG. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat Rev Endocrinol. 2023; 19:542–54.2. Midyett LK. One size fits all versus individualized medicine in type 1 diabetes management. Diabetes Technol Ther. 2023; 25(S3):S42–7.3. Yuen KC, Johannsson G, Ho KK, Miller BS, Bergada I, Rogol AD. Diagnosis and testing for growth hormone deficiency across the ages: a global view of the accuracy, caveats, and cut-offs for diagnosis. Endocr Connect. 2023; 12:e220504.4. Kerbel J, Cano-Zaragoza A, Espinosa-Dorado R, Garcia de la Torre KE, Mercado M. Real world data on the epidemiology, diagnosis, and treatment of acromegaly: a registries-based approach. Arch Med Res. 2023; 54:102856.5. Gahete MD, Cordoba-Chacon J, Lin Q, Bruning JC, Kahn CR, Castano JP, et al. Insulin and IGF-I inhibit GH synthesis and release in vitro and in vivo by separate mechanisms. Endocrinology. 2013; 154:2410–20.6. Yamamoto M, Bando H. A new insight into GH regulation and its disturbance from nutrition and autoimmune perspectives. Endocr J. 2023; 70:867–74.7. Ho KK, O’Sullivan AJ, Burt MG. The physiology of growth hormone (GH) in adults: translational journey to GH replacement therapy. J Endocrinol. 2023; 257:e220197.8. Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000; 85:4712–20.9. Al-Samerria S, Radovick S. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells. 2021; 10:2664.10. Sharma R, Kopchick JJ, Puri V, Sharma VM. Effect of growth hormone on insulin signaling. Mol Cell Endocrinol. 2020; 518:111038.11. Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002; 23:824–54.12. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006; 7:85–96.13. Shishko PI, Dreval AV, Abugova IA, Zajarny IU, Goncharov VC. Insulin-like growth factors and binding proteins in patients with recent-onset type 1 (insulin-dependent) diabetes mellitus: influence of diabetes control and intraportal insulin infusion. Diabetes Res Clin Pract. 1994; 25:1–12.14. Moller L, Dalman L, Norrelund H, Billestrup N, Frystyk J, Moller N, et al. Impact of fasting on growth hormone signaling and action in muscle and fat. J Clin Endocrinol Metab. 2009; 94:965–72.15. Blaine E, Tumlinson R, Colvin M, Haynes T, Whitley HP. Systematic literature review of insulin dose adjustments when initiating hemodialysis or peritoneal dialysis. Pharmacotherapy. 2022; 42:177–87.16. Agius R, Pace NP, Fava S. Phenotyping obesity: a focus on metabolically healthy obesity and metabolically unhealthy normal weight. Diabetes Metab Res Rev. 2023; e3725.17. Boutari C, DeMarsilis A, Mantzoros CS. Obesity and diabetes. Diabetes Res Clin Pract. 2023; 202:110773.18. Savastano S, Di Somma C, Barrea L, Colao A. The complex relationship between obesity and the somatropic axis: the long and winding road. Growth Horm IGF Res. 2014; 24:221–6.19. Nam SY, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, et al. Low-dose growth hormone treatment combined with diet restriction decreases insulin resistance by reducing visceral fat and increasing muscle mass in obese type 2 diabetic patients. Int J Obes Relat Metab Disord. 2001; 25:1101–7.20. Ahn CW, Kim CS, Nam JH, Kim HJ, Nam JS, Park JS, et al. Effects of growth hormone on insulin resistance and atherosclerotic risk factors in obese type 2 diabetic patients with poor glycaemic control. Clin Endocrinol (Oxf). 2006; 64:444–9.21. Turner RC, Holman RR. Lessons from UK prospective diabetes study. Diabetes Res Clin Pract. 1995; 28 Suppl:S151–7.22. McFarlane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep. 2009; 9:249–54.23. Marasco G, Dajti E, Ravaioli F, Brocchi S, Rossini B, Alemanni LV, et al. Clinical impact of sarcopenia assessment in patients with liver cirrhosis. Expert Rev Gastroenterol Hepatol. 2021; 15:377–88.24. Yen FS, Hou MC, Liu JS, Hsu CC, Hwu CM. Severe hypoglycemia in patients with liver cirrhosis and type 2 diabetes. Front Med (Lausanne). 2023; 9:962337.25. Assaad SN, Cunningham GR, Samaan NA. Abnormal growth hormone dynamics in chronic liver disease do not depend on severe parenchymal disease. Metabolism. 1990; 39:349–56.26. Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006; 22:830–44.27. de la Garza RG, Morales-Garza LA, Martin-Estal I, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and cirrhosis establishment. J Clin Med Res. 2017; 9:233–47.28. Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009; 119:3189–202.29. Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009; 94:1509–17.30. Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, et al. Treatment of acromegaly with the growth hormone–receptor antagonist pegvisomant. New Engl J Med. 2000; 342:1171–7.31. van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001; 358:1754–9.32. Castinetti F, Nagai M, Morange I, Dufour H, Caron P, Chanson P, et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab. 2009; 94:3400–7.33. Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009; 30:152–77.34. Jørgensen JO, Krag M, Jessen N, Norrelund H, Vestergaard ET, Moller N, et al. Growth hormone and glucose homeostasis. Horm Res. 2004; 62 Suppl 3:51–5.35. Parkinson C, Flyvbjerg A, Trainer PJ. High levels of 150-kDa insulin-like growth factor binding protein three ternary complex in patients with acromegaly and the effect of pegvisomant-induced serum IGF-I normalization. Growth Horm IGF Res. 2004; 14:59–65.36. Ho PJ, Friberg RD, Barkan AL. Regulation of pulsatile growth hormone secretion by fasting in normal subjects and patients with acromegaly. J Clin Endocrinol Metab. 1992; 75:812–9.37. Coopmans EC, Berk KA, El-Sayed N, Neggers SJ, van der Lely AJ. Eucaloric very-low-carbohydrate ketogenic diet in acromegaly treatment. N Engl J Med. 2020; 382:2161–2.38. Jorgensen JO, Rubeck KZ, Nielsen TS, Clasen BF, Vendelboe M, Hafstrom TK, et al. Effects of GH in human muscle and fat. Pediatr Nephrol. 2010; 25:705–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship of Insulin like Growth Factor I with Pharmacologically Stimulated Growth Hormone Secretion in Growth Hormone Deficient Children
- Effects of growth hormone on glucose metabolism and insulin resistance in human
- The Role of the Insulin-like Growth Factors and Insulin-like Growth Factor Binding Proteins in Growth Disorders
- Growth hormone, somatomedin C levels in umbilical cord blood in premature, term, postterm neonates
- Insulin-like growth factor I gene