J Korean Med Sci.
2024 Jan;39(1):e3. 10.3346/jkms.2024.39.e3.
Safety of COVID-19 Vaccination During Pregnancy and Lactation: A VigiBase Analysis
- Affiliations
-
- 1Department of Biohealth Regulatory Science, Sungkyunkwan University, Suwon, Korea
- 2School of Pharmacy, Sungkyunkwan University, Suwon, Korea
- 3Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea
- 4Department of Clinical Research Design & Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Korea
- KMID: 2550793
- DOI: http://doi.org/10.3346/jkms.2024.39.e3
Abstract
- Background
There is limited evidence on the safety of coronavirus disease 2019 (COVID-19) vaccination during pregnancy and lactation. Thus, we aimed to evaluate the association between COVID-19 vaccination during pregnancy and lactation and reporting risk of adverse pregnancy or lactation outcomes.
Methods
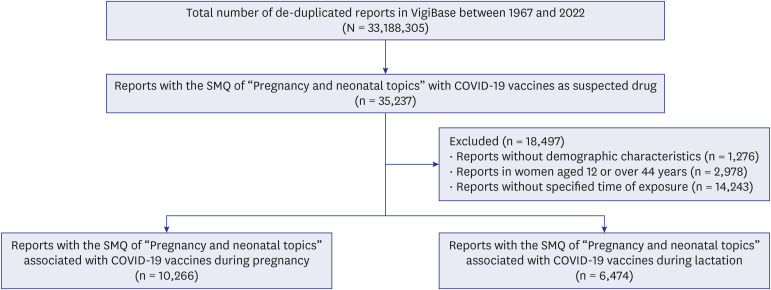
Using VigiBase, we performed a disproportionality analysis with case/non case design. Cases were defined based on the Standardized MedDRA Queries (SMQs) of “pregnancy and neonatal topics” and non-cases were defined as all other adverse events. We included all reports with COVID-19 vaccines as the suspected cause. Using the full database as the comparators, reporting odds ratios (RORs) with 95% confidence intervals (CIs) were estimated by logistic regression while adjusting for maternal age. Infants’ age and sex were additionally adjusted in analyzing the risk of COVID-19 vaccination during lactation.
Results
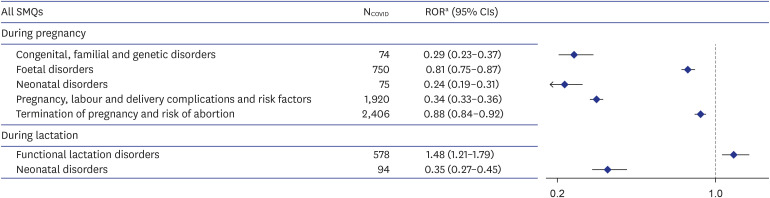
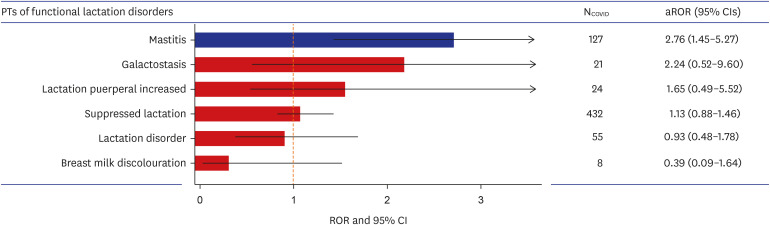
We identified 10,266 and 6,474 reports with the SMQ of “pregnancy and neonatal topics” associated with COVID-19 vaccines during pregnancy and lactation, respectively. No significant RORs of adverse pregnancy outcomes associated with COVID-19 vaccines during pregnancy were observed; however, “functional lactation disorders” showed significant disproportionality during lactation with adjusted ROR of 1.48 (95% CI, 1.21–1.79). Further analysis that analyzed “functional lactation disorders” at a preferred term level, showed higher ROR in mastitis (2.76 [95% CI, 1.45–5.27]).
Conclusion
Overall, we did not observe a positive association between COVID-19 vaccination during pregnancy and risk of reporting adverse pregnancy outcomes. However, we found a significant disproportionate reporting association between COVID-19 vaccination during lactation and “functional lactation disorders”, specifically mastitis. Continuous surveillance is warranted to confirm the safety of COVID-19 vaccine during pregnancy and lactation.
Figure
Reference
-
1. FDA. Pfizer-BioNTech COVID-19 vaccines. Updated 2023. Accessed March 28, 2023. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccines#additional .2. CDC. COVID-19 vaccine Emergency Use Authorization (EUA) fact sheets for recipients and caregivers. Updated 2021. Accessed March 28, 2023. https://www.cdc.gov/vaccines/covid-19/eua/index.html .3. Park WB, Hwang YH, Cheong HJ. COVID-19 vaccination in Korea. Infect Chemother. 2023; 55(1):135–149. PMID: 37021429.4. Hong SH, Shi HJ, Kim SY, Park Y, Eom JS. Clinical characteristics and pregnancy-related outcomes of pregnant women hospitalized with COVID-19 during the delta wave: a single-center observational study. Infect Chemother. 2022; 54(3):433–445. PMID: 35920268.5. Badell ML, Dude CM, Rasmussen SA, Jamieson DJ. COVID-19 vaccination in pregnancy. BMJ. 2022; 378:e069741. PMID: 35948352.6. Ahn KH, Kim HI, Lee KS, Heo JS, Kim HY, Cho GJ, et al. COVID-19 and vaccination during pregnancy: a systematic analysis using Korea National Health Insurance claims data. Obstet Gynecol Sci. 2022; 65(6):487–501. PMID: 35916014.7. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020; 370:m3320. PMID: 32873575.8. Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020; 323(18):1848–1849. PMID: 32215589.9. Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn. 2020; 40(13):1759–1761. PMID: 32304114.10. CDC. Precautions for people with certain medical conditions. Updated 2023. Accessed March 28, 2023. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html .11. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021; 224(1):35–53.e3. PMID: 32739398.12. Lee J, Lee MY, Lee J, Jang E, Bae S, Jung J, et al. Clinical characteristics and vertical transmission of severe acute respiratory syndrome coronavirus 2 infection in pregnant women and their neonates in Korea. Infect Chemother. 2023; 55(3):346–354. PMID: 37503777.13. CDC. COVID-19 vaccines while pregnant or brestfeeding. Updated 2023. Accessed October 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html .14. COVID-19 vaccination: a guide on pregnancy and breastfeeding. Updated 2023. Accessed March 28, 2023. https://www.gov.uk/government/publications/covid-19-vaccination-women-of-childbearing-age-currently-pregnant-planning-a-pregnancy-or-breastfeeding/covid-19-vaccination-a-guide-on-pregnancy-and-breastfeeding#breastfeeding .15. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021; 384(24):2273–2282. PMID: 33882218.16. Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. COVID-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021; 385(21):2008–2010. PMID: 34670062.17. Kharbanda EO, Haapala J, DeSilva M, Vazquez-Benitez G, Vesco KK, Naleway AL, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021; 326(16):1629–1631. PMID: 34495304.18. Rimmer MP, Teh JJ, Mackenzie SC, Al Wattar BH. The risk of miscarriage following COVID-19 vaccination: a systematic review and meta-analysis. Hum Reprod. 2023; 38(5):840–852. PMID: 36794918.19. Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth - eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022; 71(1):26–30. PMID: 34990445.20. Karasek D, Baer RJ, McLemore MR, Bell AJ, Blebu BE, Casey JA, et al. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Health Am. 2021; 2:100027. PMID: 34642685.21. Muyldermans J, De Weerdt L, De Brabandere L, Maertens K, Tommelein E. The effects of COVID-19 vaccination on lactating women: a systematic review of the literature. Front Immunol. 2022; 13:852928. PMID: 35464406.22. Hamid AAA, Rahim R, Teo SP. Pharmacovigilance and its importance for primary health care professionals. Korean J Fam Med. 2022; 43(5):290–295. PMID: 36168900.23. WHO-UMC. About VigiBase. Updated 2023. Accessed March 28, 2023. https://who-umc.org/vigibase/ .24. MedDRA. Standardised MedDRA Queries (SMQs). Updated 2023. Accessed March 28, 2023. https://www.meddra.org/how-to-use/tools/smqs .25. EMA. Screening for adverse reactions in Eudravigilance. Updated 2016. Accessed March 28, 2023. https://www.ema.europa.eu/en/documents/other/screening-adverse-reactions-eudravigilance_en.pdf .26. EMA. Guideline on good pharmacovigilance practices (GVP): module IX addendum I - methodological aspects of signal detection from spontaneous reports of suspected adverse reactions. Updated 2017. Accessed October 6, 2023. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-ix-addendum-i-methodological-aspects-signal_en.pdf .27. Sperling RS, Riley LE. Immunization and Emerging Infections Expert Work Group. Influenza vaccination, pregnancy safety, and risk of early pregnancy loss. Obstet Gynecol. 2018; 131(5):799–802. PMID: 29630014.28. CDC. Flu vaccine safety and pregnancy. Updated 2022. Accessed March 28, 2023. https://www.cdc.gov/flu/highrisk/qa_vacpregnant.htm .29. Murray D. Breastfeeding and the flu vaccine. Updated 2022. Accessed March 28, 2023. https://www.verywellfamily.com/breastfeeding-and-the-flu-vaccine-431604 .30. Brady RC, Jackson LA, Frey SE, Shane AL, Walter EB, Swamy GK, et al. Randomized trial comparing the safety and antibody responses to live attenuated versus inactivated influenza vaccine when administered to breastfeeding women. Vaccine. 2018; 36(31):4663–4671. PMID: 29961606.31. Yoon H, Choi BY, Seong WJ, Cho GJ, Na S, Jung YM, et al. COVID-19 vaccine acceptance during pregnancy and influencing factors in South Korea. J Clin Med. 2022; 11(19):5733. PMID: 36233601.32. Blumberg D, Sridhar A, Lakshminrusimha S, Higgins RD, Saade G. COVID-19 vaccine considerations during pregnancy and lactation. Am J Perinatol. 2021; 38(6):523–528. PMID: 33932943.33. Prasad S, Kalafat E, Blakeway H, Townsend R, O'Brien P, Morris E, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. 2022; 13(1):2414. PMID: 35538060.34. Magnus MC, Örtqvist AK, Dahlqwist E, Ljung R, Skår F, Oakley L, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022; 327(15):1469–1477. PMID: 35323851.35. Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022; 327(15):1478–1487. PMID: 35323842.36. KCDC. Causality Assessment of Adverse Events Following COVID-19 Vaccination. Cheongju, Korea: KCDC;2021.37. EMA. COVID-19: latest safety data provide reassurance about use of mRNA vaccines during pregnancy. Updated 2022. Accessed August 16, 2023. https://www.ema.europa.eu/en/news/covid-19-latest-safety-data-provide-reassurance-about-use-mrna-vaccines-during-pregnancy .38. England PH. JCVI issues new advice on COVID-19 vaccination for pregnant women. Updated 2021. Accessed September 12, 2023. https://www.gov.uk/government/news/jcvi-issues-new-advice-on-covid-19-vaccination-for-pregnant-women .39. Australian Government. Pregnancy, breastfeeding and COVID-19 vaccines. Updated 2023. Accessed October 6, 2023. https://www.health.gov.au/our-work/covid-19-vaccines/who-can-get-vaccinated/pregnant-women .40. WHO. COVID-19 advice for the public: getting vaccinated. Updated 2023. Accessed October 6, 2023. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice .41. Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021; 4(8):e2121310. PMID: 34402893.42. McLaurin-Jiang S, Garner CD, Krutsch K, Hale TW. Maternal and child symptoms following COVID-19 vaccination among breastfeeding mothers. Breastfeed Med. 2021; 16(9):702–709. PMID: 34171971.43. Low JM, Lee LY, Ng YP, Zhong Y, Amin Z. Breastfeeding mother and child clinical outcomes after COVID-19 vaccination. J Hum Lact. 2022; 38(1):37–42. PMID: 34713745.44. Eurorad. Post COVID-19 vaccination mastitis. Updated 2021. Accessed March 28, 2023. https://www.eurorad.org/case/17342 .45. CDC. COVID-19 vaccines while pregnant or breastfeeding. Updated 2022. Accessed March 28, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html .46. WHO. Questions and answers: COVID-19 vaccines and pregnancy. Updated 2022. Accessed March 28, 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-FAQ-Pregnancy-Vaccines-2022.1 .47. Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021; 326(8):728–735. PMID: 34251417.48. Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021; 325(23):2370–2380. PMID: 33983379.49. Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011; 72(6):905–908. PMID: 21658092.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Events Following COVID-19 Vaccination in Adolescents: Insights From Pharmacovigilance Study of VigiBase
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- COVID-19 and vaccination during pregnancy: a systematic analysis using Korea National Health Insurance claims data
- Factors Influencing the COVID-19 Vaccination Intentions in Nurses: Korea, February 2021
- Coronavirus Disease 2019 Vaccination during Pregnancy