Korean J Physiol Pharmacol.
2024 Jan;28(1):39-48. 10.4196/kjpp.2024.28.1.39.
Wogonin attenuates vascular remodeling by inhibiting smooth muscle cell proliferation and migration in hypertensive rat
- Affiliations
-
- 1Department of Cardiovasology, The First Affiliated Hospital, Hainan Medical University, Haikou 570100, China
- 2Hainan Eye Hospital and Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Haikou 570311, China
- 3Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430014, China
- KMID: 2550287
- DOI: http://doi.org/10.4196/kjpp.2024.28.1.39
Abstract
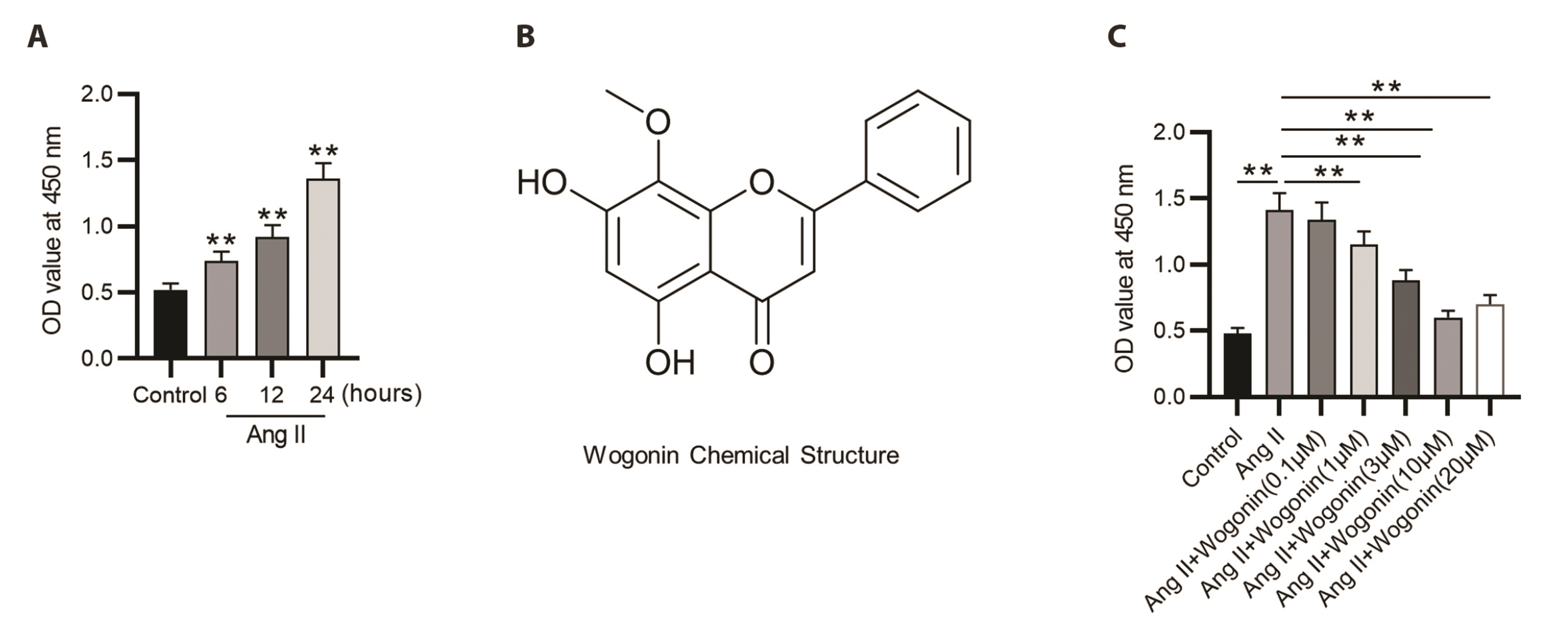
- Wogonin, extracted from the roots of Scutellaria baicalensis Georgi, has been shown to suppress collagen deposition in spontaneously hypertensive rats (SHRs). This study was performed to investigate the role and mechanism of wogonin underlying vascular remodeling in SHRs. After injection of SHRs with 40 mg/kg of wogonin, blood pressure in rats was measured once a week. Masson's trichrome staining was conducted to observe the changes in aortas and mesenteric arteries. Vascular smooth muscle cells (VSMCs) isolated from rat thoracic aortas were treated with Angiotensin II (Ang II; 100 nM) in the presence or absence of varying concentrations of wogonin. The viability and proliferation of VSMCs were examined using Cell Counting Kit-8 assay and 5-ethynyl-2’-deoxyuridine assay, respectively. The migration of VSMCs was examined using wound healing assay and transwell assay. We found that wogonin administration alleviated hypertension, increased lumen diameter, and reduced the thickness of the arterial media in SHRs. Ang II treatment enhanced the viability of VSMCs, which was inhibited by wogonin in a concentration-dependent manner. Wogonin reversed Ang II-induced increases in the viability, proliferation, and migration of VSMCs. Moreover, wogonin inhibited Ang II-induced activation of mitogen-activated protein kinase (MAPK) signaling in VSMCs. Overall, wogonin repressed the proliferative and migratory capacity of VSMCs by regulating the MAPK signaling pathway, thereby attenuating vascular remodeling in hypertensive rats, indicating that wogonin might be a therapeutic agent for the treatment of vascular diseases.
Keyword
Figure
Reference
-
1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. 2005; Global burden of hypertension: analysis of worldwide data. Lancet. 365:217–223. DOI: 10.1016/S0140-6736(05)17741-1. PMID: 15652604.2. Xu MM, Deng HY, Li HH. 2019; MicroRNA-27a regulates angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting α-smooth muscle-actin in vitro. Biochem Biophys Res Commun. 509:973–977. DOI: 10.1016/j.bbrc.2019.01.047. PMID: 30654940.3. Rizzoni D, Agabiti Rosei E. 2006; Small artery remodeling in hypertension and diabetes. Curr Hypertens Rep. 8:90–95. DOI: 10.1007/s11906-006-0046-3. PMID: 16600165.4. Wang D, Uhrin P, Mocan A, Waltenberger B, Breuss JM, Tewari D, Mihaly-Bison J, Huminiecki Ł, Starzyński RR, Tzvetkov NT, Horbańczuk J, Atanasov AG. 2018; Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: molecular targets and pathways. Biotechnol Adv. 36:1586–1607. DOI: 10.1016/j.biotechadv.2018.04.006. PMID: 29684502.5. Schiffrin EL. 2012; Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 59:367–374. DOI: 10.1161/HYPERTENSIONAHA.111.187021. PMID: 22203749.6. Mendoza-Torres E, Oyarzún A, Mondaca-Ruff D, Azocar A, Castro PF, Jalil JE, Chiong M, Lavandero S, Ocaranza MP. 2015; ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis. 9:217–237. DOI: 10.1177/1753944715597623. PMID: 26275770.7. Touyz RM. 2005; Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 90:449–455. DOI: 10.1113/expphysiol.2005.030080. PMID: 15890798.8. Dai B, Wang ZZ, Zhang H, Han MX, Zhang GX, Chen JW. 2020; Antihypertensive properties of a traditional Chinese medicine GAO-ZI-YAO in elderly spontaneous hypertensive rats. Biomed Pharmacother. 131:110739. DOI: 10.1016/j.biopha.2020.110739. PMID: 32932045.9. Liu L, Shi Q, Liu X, Li Y, Li X. 2022; Attenuation of myocardial fibrosis using molecular hydrogen by inhibiting the TGF-β signaling pathway in spontaneous hypertensive rats. Am J Hypertens. 35:156–163. DOI: 10.1093/ajh/hpab159. PMID: 34618887.10. Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. 2014; Angiotensin II and vascular injury. Curr Hypertens Rep. 16:431. DOI: 10.1007/s11906-014-0431-2. PMID: 24760441.11. Mehta PK, Griendling KK. 2007; Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97. DOI: 10.1152/ajpcell.00287.2006. PMID: 16870827.12. Shen A, Wu M, Ali F, Guo Z, Fang Y, Zhou Y, Zhang S, Zhang W, Wen Y, Yu M, Peng J, Chen K. 2023; Based on network pharmacology, gastrodin attenuates hypertension-induced vascular smooth muscle cell proliferation and PI3K/AKT pathway activation. Sci Rep. 13:12140. DOI: 10.1038/s41598-023-39202-6. PMID: 37495624. PMCID: PMC10372005. PMID: 6e734266f8a6410589aa43e8f8341890.13. Ye C, Geng Z, Zhang LL, Zheng F, Zhou YB, Zhu GQ, Xiong XQ. 2022; Chronic infusion of ELABELA alleviates vascular remodeling in spontaneously hypertensive rats via anti-inflammatory, anti-oxidative and anti-proliferative effects. Acta Pharmacol Sin. 43:2573–2584. DOI: 10.1038/s41401-022-00875-w. PMID: 35260820. PMCID: PMC9525578.14. Wei CY, Sun HL, Yang ML, Yang CP, Chen LY, Li YC, Lee CY, Kuan YH. 2017; Protective effect of wogonin on endotoxin-induced acute lung injury via reduction of p38 MAPK and JNK phosphorylation. Environ Toxicol. 32:397–403. DOI: 10.1002/tox.22243. PMID: 26892447.15. Baumann S, Fas SC, Giaisi M, Müller WW, Merling A, Gülow K, Edler L, Krammer PH, Li-Weber M. 2008; Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCγ1- and Ca2+-dependent apoptosis. Blood. 111:2354–2363. DOI: 10.1182/blood-2007-06-096198. PMID: 18070986.16. Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ, Choi WS, Suk K. 2003; Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 17:1943–1944. DOI: 10.1096/fj.03-0057fje. PMID: 12897065.17. Chi YS, Cheon BS, Kim HP. 2001; Effect of wogonin, a plant flavone from Scutellaria radix, on the suppression of cyclooxygenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW 264.7 cells. Biochem Pharmacol. 61:1195–1203. DOI: 10.1016/S0006-2952(01)00597-4. PMID: 11322923.18. Huang HC, Wang HR, Hsieh LM. 1994; Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur J Pharmacol. 251:91–93. DOI: 10.1016/0014-2999(94)90447-2. PMID: 8137874.19. Lee SO, Jeong YJ, Yu MH, Lee JW, Hwangbo MH, Kim CH, Lee IS. 2006; Wogonin suppresses TNF-alpha-induced MMP-9 expression by blocking the NF-kappaB activation via MAPK signaling pathways in human aortic smooth muscle cells. Biochem Biophys Res Commun. 351:118–125. DOI: 10.1016/j.bbrc.2006.10.006. PMID: 17052690.20. Liu YM, Wang X, Nawaz A, Kong ZH, Hong Y, Wang CH, Zhang JJ. 2011; Wogonin ameliorates lipotoxicity-induced apoptosis of cultured vascular smooth muscle cells via interfering with DAG-PKC pathway. Acta Pharmacol Sin. 32:1475–1482. DOI: 10.1038/aps.2011.120. PMID: 21986573. PMCID: PMC4010212.21. Wang SJ, Zhao JK, Ren S, Sun WW, Zhang WJ, Zhang JN. 2019; Wogonin affects proliferation and the energy metabolism of SGC-7901 and A549 cells. Exp Ther Med. 17:911–918. DOI: 10.3892/etm.2018.7023. PMID: 30651880. PMCID: PMC6307470.22. Lin CM, Chang H, Chen YH, Wu IH, Chiu JH. 2006; Wogonin inhibits IL-6-induced angiogenesis via down-regulation of VEGF and VEGFR-1, not VEGFR-2. Planta Med. 72:1305–1310. DOI: 10.1055/s-2006-951692. PMID: 17024605.23. Kong EK, Huang Y, Sanderson JE, Chan KB, Yu S, Yu CM. 2010; Baicalein and Wogonin inhibit collagen deposition in SHR and WKY cardiac fibroblast cultures. BMB Rep. 43:297–303. DOI: 10.5483/BMBRep.2010.43.4.297. PMID: 20423617.24. Qu JT, Zhang DX, Liu F, Mao HP, Ma YK, Yang Y, Li CX, Qiu LZ, Geng X, Zhang JM, Gao XM, Chen L, Wang H. 2015; Vasodilatory effect of wogonin on the rat aorta and its mechanism study. Biol Pharm Bull. 38:1873–1878. DOI: 10.1248/bpb.b15-00444. PMID: 26632179.25. Xu J, Zhang B, Chu Z, Jiang F, Han J. 2021; Wogonin alleviates cisplatin-induced cardiotoxicity in mice via inhibiting Gasdermin D-mediated pyroptosis. J Cardiovasc Pharmacol. 78:597–603. DOI: 10.1097/FJC.0000000000001085. PMID: 34651602. PMCID: PMC8492184.26. Decean HP, Brie IC, Tatomir CB, Perde-Schrepler M, Fischer-Fodor E, Virag P. 2018; Targeting MAPK (p38, ERK, JNK) and inflammatory CK (GDF-15, GM-CSF) in UVB-activated human skin cells with Vitis vinifera seed extract. J Environ Pathol Toxicol Oncol. 37:261–272. DOI: 10.1615/JEnvironPatholToxicolOncol.2018027009. PMID: 30317975.27. Cao X, Fang X, Guo M, Li X, He Y, Xie M, Xu Y, Liu X. 2021; TRB3 mediates vascular remodeling by activating the MAPK signaling pathway in hypoxic pulmonary hypertension. Respir Res. 22:312. DOI: 10.1186/s12931-021-01908-4. PMID: 34906150. PMCID: PMC8670293. PMID: cd5268d388f74d458e3317039598cf1f.28. Talbi A, Zhao D, Liu Q, Li J, Fan A, Yang W, Han X, Chen X. 2014; Pharmacokinetics, tissue distribution, excretion and plasma protein binding studies of wogonin in rats. Molecules. 19:5538–5549. DOI: 10.3390/molecules19055538. PMID: 24786691. PMCID: PMC6270787. PMID: 68472b83616a4e2092e0e4593dcb45d7.29. Tian L, Cai D, Zhuang D, Wang W, Wang X, Bian X, Xu R, Wu G. 2020; miR-96-5p regulates proliferation, migration, and apoptosis of vascular smooth muscle cell induced by angiotensin II via targeting NFAT5. J Vasc Res. 57:86–96. DOI: 10.1159/000505457. PMID: 32045906.30. Yang H, Liu J, Chen X, Li G. 2022; Angptl2 gene knockdown is critical for abolishing angiotensin II-induced vascular smooth muscle cell proliferation and migration. Biochem Cell Biol. 100:59–67. DOI: 10.1139/bcb-2021-0191. PMID: 34860608.31. Zhang Y, Qian X, Sun X, Lin C, Jing Y, Yao Y, Ma Z, Kuai M, Lu Y, Kong X, Chen Q, Wu X, Zhao X, Li Y, Bian H. 2018; Liuwei Dihuang, a traditional Chinese medicinal formula, inhibits proliferation and migration of vascular smooth muscle cells via modulation of estrogen receptors. Int J Mol Med. 42:31–40. DOI: 10.3892/ijmm.2018.3622. PMID: 29693116. PMCID: PMC5979928.32. Kang G, Lee YR, Joo HK, Park MS, Kim CS, Choi S, Jeon BH. 2015; Trichostatin A modulates angiotensin II-induced vasoconstriction and blood pressure via inhibition of p66shc activation. Korean J Physiol Pharmacol. 19:467–472. DOI: 10.4196/kjpp.2015.19.5.467. PMID: 26330760. PMCID: PMC4553407.33. Zhong X, Ma Z, Su Y, Li Z, Liao Y, Pan X, Zang L, Zhou S. 2020; Flavin adenine dinucleotide ameliorates hypertensive vascular remodeling via activating short chain acyl-CoA dehydrogenase. Life Sci. 258:118156. DOI: 10.1016/j.lfs.2020.118156. PMID: 32735886.34. Gao Y, Ren C, Li X, Yu W, Li S, Li H, Wang Y, Li D, Ren M, Ji X. 2021; Ischemic conditioning ameliorated hypertension and vascular remodeling of spontaneously hypertensive rat via inflammatory regulation. Aging Dis. 12:116–131. DOI: 10.14336/AD.2020.0320. PMID: 33532132. PMCID: PMC7801289.35. Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY, Liao X, Sun HJ. 2018; Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 14:656–668. DOI: 10.1016/j.redox.2017.11.012. PMID: 29175753. PMCID: PMC5716955.36. He L, Zhou Q, Huang Z, Xu J, Zhou H, Lv D, Lu L, Huang S, Tang M, Zhong J, Chen JX, Luo X, Li L, Chen L. 2019; PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKα and exacerbates atherosclerotic lesions. J Cell Physiol. 234:8668–8682. Erratum. DOI: 10.1002/jcp.27527. PMID: 30456860.37. Huynh DTN, Heo KS. 2021; Role of mitochondrial dynamics and mitophagy of vascular smooth muscle cell proliferation and migration in progression of atherosclerosis. Arch Pharm Res. 44:1051–1061. DOI: 10.1007/s12272-021-01360-4. PMID: 34743301.38. Shi N, Chen SY. 2016; Smooth muscle cell differentiation: model systems, regulatory mechanisms, and vascular diseases. J Cell Physiol. 231:777–787. DOI: 10.1002/jcp.25208. PMID: 26425843.39. Zhao XS, Zheng B, Wen Y, Sun Y, Wen JK, Zhang XH. 2019; Salvianolic acid B inhibits Ang II-induced VSMC proliferation in vitro and intimal hyperplasia in vivo by downregulating miR-146a expression. Phytomedicine. 58:152754. DOI: 10.1016/j.phymed.2018.11.014. PMID: 31009837.40. Hong M, Cheng H, Song L, Wang W, Wang Q, Xu D, Xing W. 2018; Wogonin suppresses the activity of matrix metalloproteinase-9 and inhibits migration and invasion in human hepatocellular carcinoma. Molecules. 23:384. DOI: 10.3390/molecules23020384. PMID: 29439451. PMCID: PMC6017513. PMID: 124e68c7eb1445d5a3770879f7558bd1.41. Wang XH, Wei YF, Cheng H. 2016; Effects of Wogonin on apoptosis, invasion, migration and Wnt/β-catenin signaling pathway of gastric cancer cells SGC7901. Zhong Yao Cai. 39:1372–1376. Chinese. PMID: 30156810.42. Li L, Ji Y, Zhang L, Cai H, Ji Z, Gu L, Yang S. 2021; Wogonin inhibits the growth of HT144 melanoma via regulating hedgehog signaling-mediated inflammation and glycolysis. Int Immunopharmacol. 101:108222. DOI: 10.1016/j.intimp.2021.108222. PMID: 34688155.43. Wang Y, Chen S, Sun S, Liu G, Chen L, Xia Y, Cui J, Wang W, Jiang X, Zhang L, Zhu Y, Zou Y, Shi B. 2020; Wogonin induces apoptosis and reverses sunitinib resistance of renal cell carcinoma cells via inhibiting CDK4-RB pathway. Front Pharmacol. 11:1152. DOI: 10.3389/fphar.2020.01152. PMID: 32792963. PMCID: PMC7394056. PMID: 667947bfe28642028a7128837ca2bc1b.44. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. 2015; Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 35:600–604. DOI: 10.3109/10799893.2015.1030412. PMID: 26096166.45. Liu Y, Sun Z, Zhu J, Xiao B, Dong J, Li X. 2018; LncRNA-TCONS_00034812 in cell proliferation and apoptosis of pulmonary artery smooth muscle cells and its mechanism. J Cell Physiol. 233:4801–4814. DOI: 10.1002/jcp.26279. PMID: 29150946.46. Chen T, Su S, Yang Z, Zhang D, Li Z, Lu D. 2022; Srolo Bzhtang reduces inflammation and vascular remodeling via suppression of the MAPK/NF-κB signaling pathway in rats with pulmonary arterial hypertension. J Ethnopharmacol. 297:115572. DOI: 10.1016/j.jep.2022.115572. PMID: 35872290.47. Fang W, Zhou X, Wang J, Xu L, Zhou L, Yu W, Tao Y, Zhu J, Hu B, Liang C, Li F, Hua J, Chen Q. 2018; Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int Immunopharmacol. 65:539–549. DOI: 10.1016/j.intimp.2018.10.024. PMID: 30412851.48. Huang Y, Guo L, Chitti R, eeharsha N Sr, Mishra A, Gubbiyappa SK, Singh Y. 2020; Wogonin ameliorate complete Freund's adjuvant induced rheumatoid arthritis via targeting NF-κB/MAPK signaling pathway. Biofactors. 46:283–291. DOI: 10.1002/biof.1585. PMID: 31721330.49. Du Y, Chen XY, Yang HY, Zhong DF. 2002; Determination of wogonin in rat plasma by liquid chromatography-tandem mass spectrometry. Yao Xue Xue Bao. 37:362–366. Chinese. PMID: 12579842.50. Peng J, Qi Q, You Q, Hu R, Liu W, Feng F, Wang G, Guo Q. 2009; Subchronic toxicity and plasma pharmacokinetic studies on wogonin, a natural flavonoid, in Beagle dogs. J Ethnopharmacol. 124:257–262. DOI: 10.1016/j.jep.2009.04.031. PMID: 19397969.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of anti-hypertensive drugs on DNA synthesis and proliferation of ultured rat aortic smooth muscle cells
- Effect of Carvedilol Alone or with Cyclosporine on the Migration of Cultured Rat Vascular Smooth Muscle Cell
- The Effect of High Glucose on the Proliferation and Migration of Vascular Smooth Muscle Cells
- Intimal Hyperplasia
- Pitavastatin Regulates Ang II Induced Proliferation and Migration via IGFBP-5 in VSMC