Ann Rehabil Med.
2023 Dec;47(6):483-492. 10.5535/arm.23138.
Preclinical Study of Dual-Wavelength Light-Emitting Diode Therapy in an Osteoarthritis Rat Model
- Affiliations
-
- 1Department of Rehabilitation Medicine, Wonju Severance Christian Hospital, Wonju, Korea
- 2Yonsei Institute of Sports Science and Exercise Medicine, Wonju, Korea
- 3Department of Biomedical Engineering, Yonsei University, Wonju, Korea
- 4Research Institute of Hyperbaric Medicine and Science, Yonsei University Wonju College of Medicine, Wonju, Korea
- KMID: 2549729
- DOI: http://doi.org/10.5535/arm.23138
Abstract
Objective
To evaluate the efficacy of light-emitting diode (LED) and their dual-wavelengths as a treatment strategy for osteoarthritis.
Methods
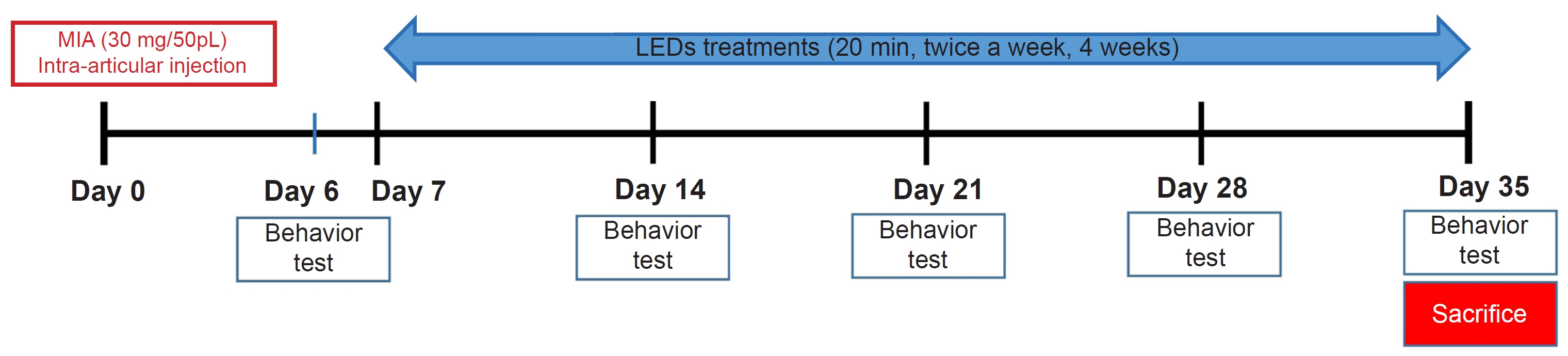
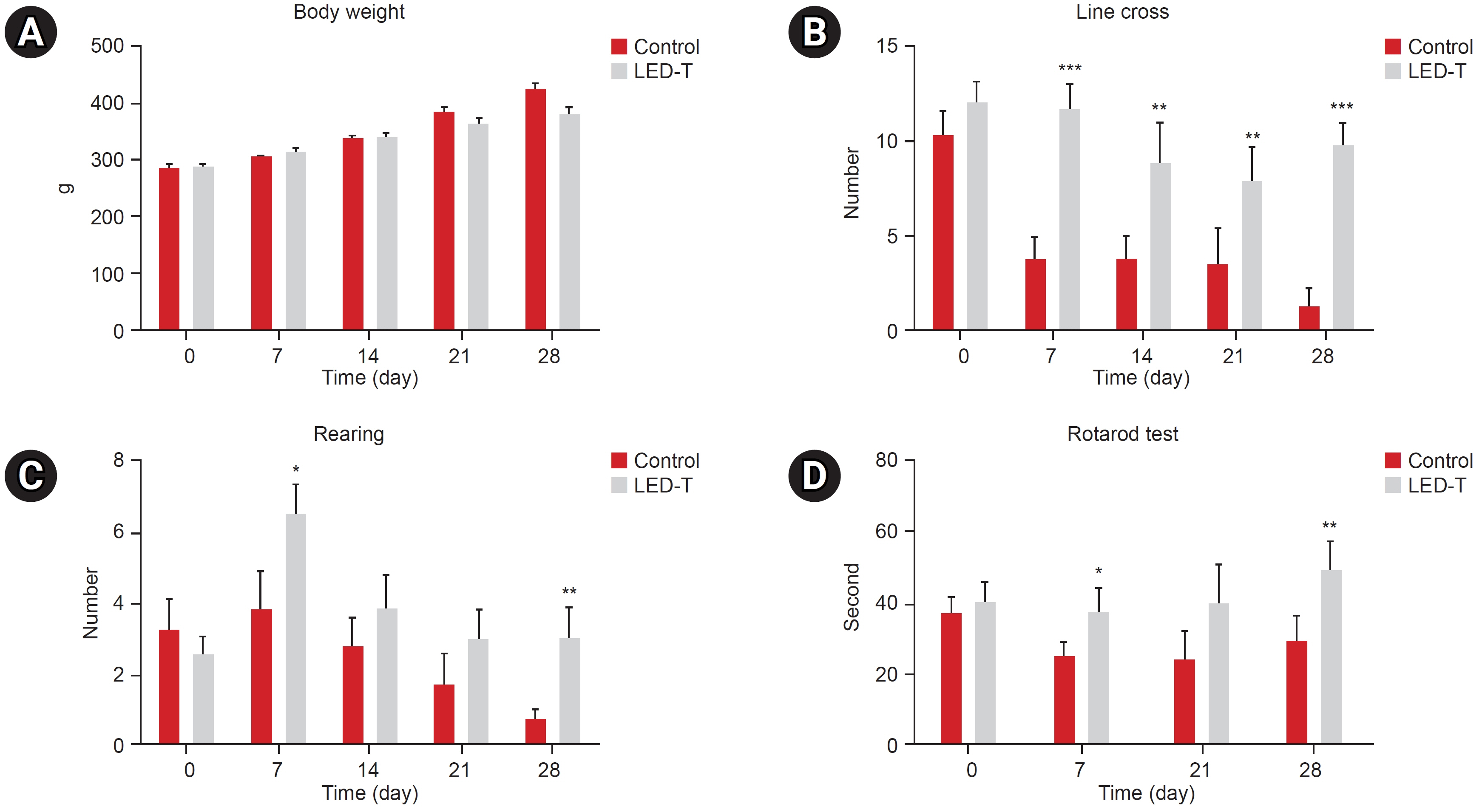
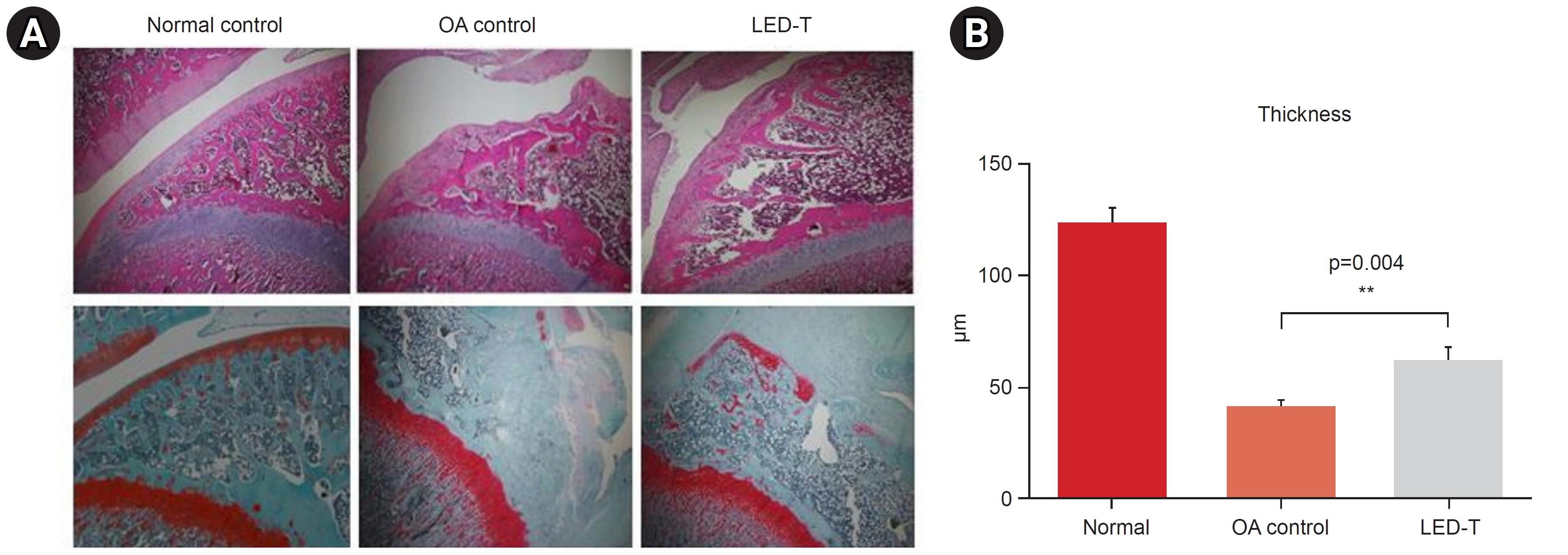
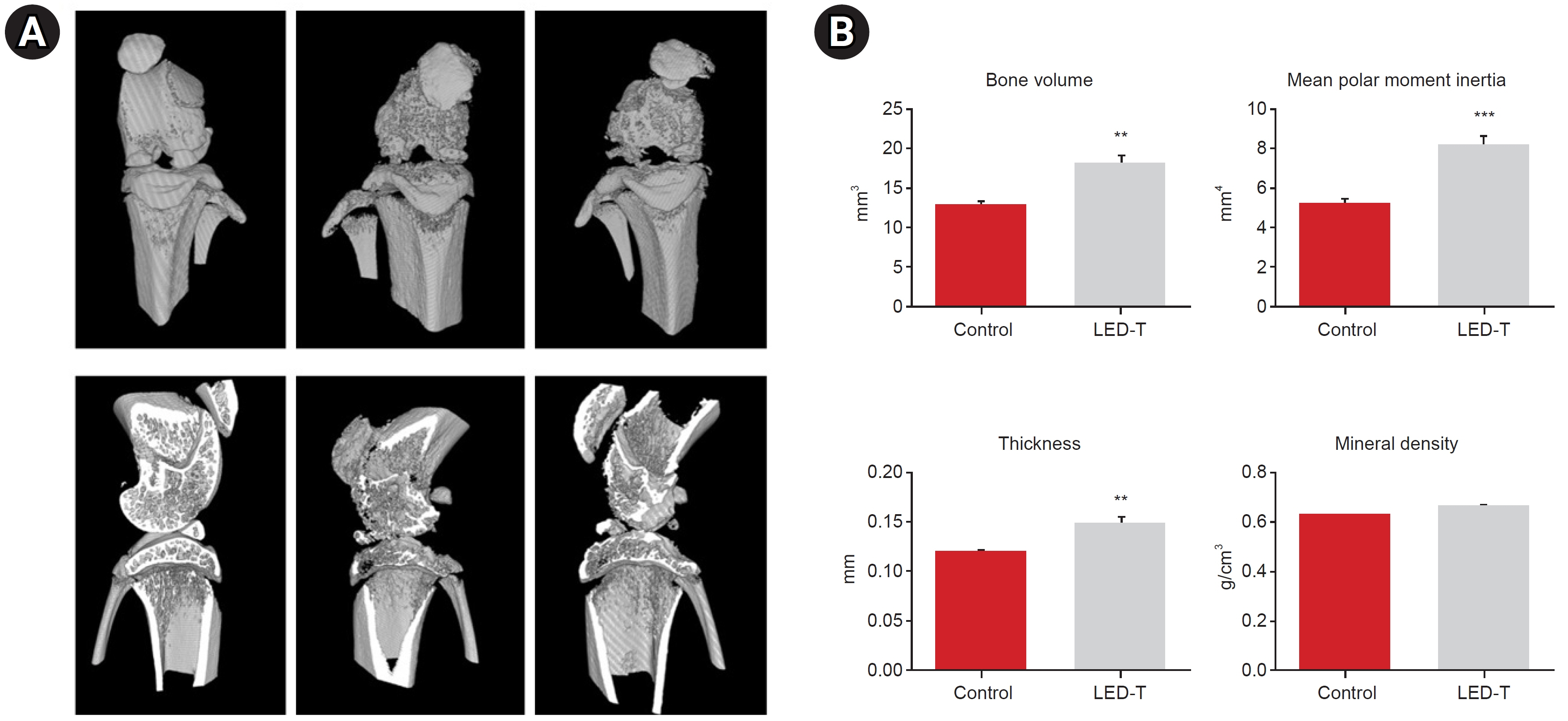
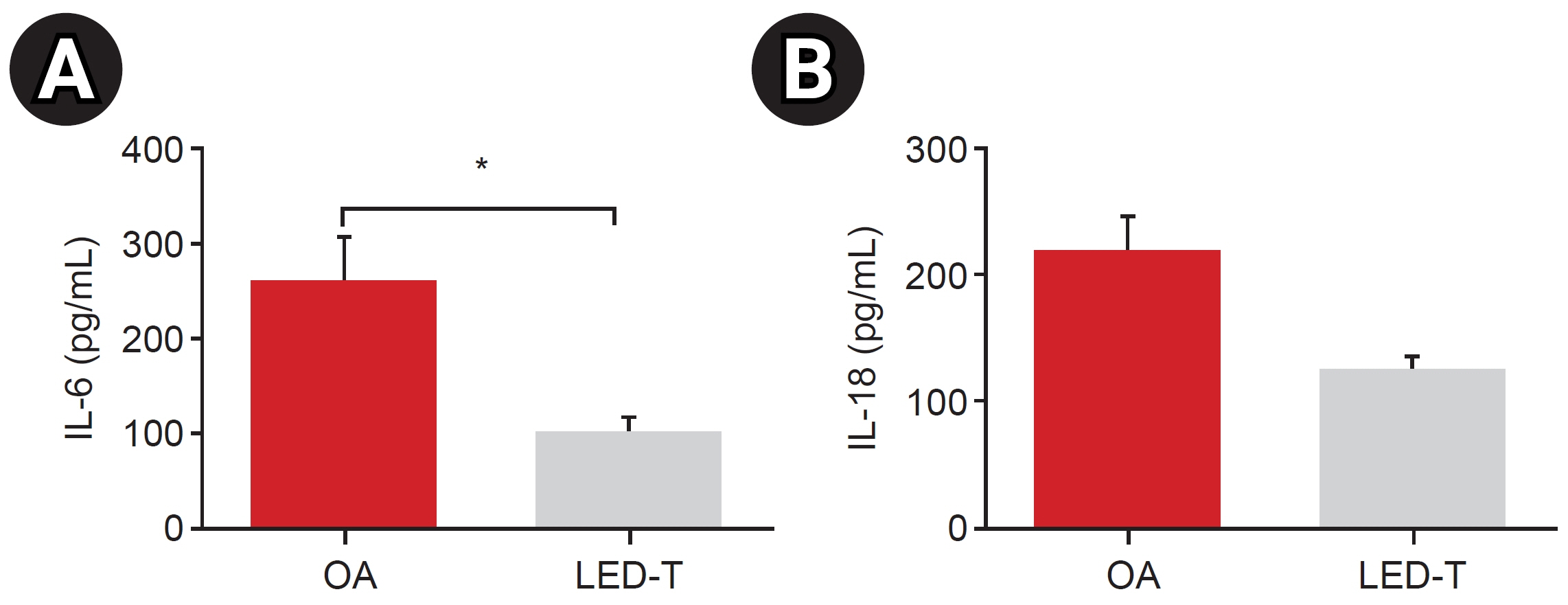
We induced osteoarthritis in male Sprague-Dawley rats by intra-articular injection of sodium iodoacetate into the right rear knee joint. The animals with lesions were divided into an untreated group and an LED-treated group (n=7 each). In the LED-treated group, the lesioned knee was irradiated with lasers (850 and 940 nm) and dose (3.15 J/cm2) for 20 minutes per session, twice a week for 4 weeks. Knee joint tissues were stained and scanned using an in vivo micro-computed tomography (CT) scanner. Serum interleukin (IL)-6 and IL-18 levels were determined using enzyme-linked immuno-sorbent assay. Several functional tests (lines crossed, rotational movement, rearing, and latency to remain rotating rod) were performed 24 hours before LED treatment and at 7, 14, 21, and 28 days after treatment.
Results
LED-treated rats showed improved locomotor function and suppressed matrix-degrading cytokines. Micro-CT images indicated that LED therapy had a preserving effect on cartilage and cortical bone.
Conclusion
LED treatment using wavelengths of 850 and 940 nm resulted in significant functional, anatomical, and histologic improvements without adverse events in a rat model. Further research is required to determine the optimal wavelength, duration, and combination method, which will maximize treatment effectiveness.
Keyword
Figure
Reference
-
1. Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008; 22:351–84.
Article2. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019; 393:1745–59.
Article3. Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012; 85:49–56. Erratum in: Am Fam Physician 2012;86:893.4. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020; 180:114147.
Article5. Mou D, Yu Q, Zhang J, Zhou J, Li X, Zhuang W, et al. Intra-articular injection of chitosan-based supramolecular hydrogel for osteoarthritis treatment. Tissue Eng Regen Med. 2021; 18:113–25.
Article6. Rahimi M, Charmi G, Matyjaszewski K, Banquy X, Pietrasik J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021; 123:31–50.
Article7. Quinn RH, Murray JN, Pezold R, Sevarino KS. Surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2018; 26:e191–3.
Article8. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009; 374:1897–908. Erratum in: Lancet 2010;375:894.
Article9. Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971; 122:532–5.
Article10. Mussttaf RA, Jenkins DFL, Jha AN. Assessing the impact of low level laser therapy (LLLT) on biological systems: a review. Int J Radiat Biol. 2019; 95:120–43.
Article11. Rochkind S, Geuna S, Shainberg A. Chapter 25: phototherapy in peripheral nerve injury: effects on muscle preservation and nerve regeneration. Int Rev Neurobiol. 2009; 87:445–64.12. Tchanque-Fossuo CN, Ho D, Dahle SE, Koo E, Li CS, Isseroff RR, et al. A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen. 2016; 24:418–26.
Article13. DE Oliveira MF, Johnson DS, Demchak T, Tomazoni SS, Leal-Junior EC. Low-intensity LASER and LED (photobiomodulation therapy) for pain control of the most common musculoskeletal conditions. Eur J Phys Rehabil Med. 2022; 58:282–9.
Article14. de Boer E, Warram JM, Hartmans E, Bremer PJ, Bijl B, Crane LM, et al. A standardized light-emitting diode device for photoimmunotherapy. J Nucl Med. 2014; 55:1893–8.
Article15. Barolet AC, Villarreal AM, Jfri A, Litvinov IV, Barolet D. Low-intensity visible and near-infrared light-induced cell signaling pathways in the skin: a comprehensive review. Photobiomodul Photomed Laser Surg. 2023; 41:147–66.
Article16. Hamblin MR, Liebert A. Photobiomodulation therapy mechanisms beyond cytochrome c oxidase. Photobiomodul Photomed Laser Surg. 2022; 40:75–7.
Article17. Silveira PC, Ferreira KB, da Rocha FR, Pieri BL, Pedroso GS, De Souza CT, et al. Effect of low-power laser (LPL) and light-emitting diode (LED) on inflammatory response in burn wound healing. Inflammation. 2016; 39:1395–404.
Article18. Belém MO, de Andrade GMM, Carlos TM, Guazelli CFS, Fattori V, Toginho Filho DO, et al. Light-emitting diodes at 940nm attenuate colitis-induced inflammatory process in mice. J Photochem Photobiol B. 2016; 162:367–73.19. Goldberg DJ, Amin S, Russell BA, Phelps R, Kellett N, Reilly LA. Combined 633-nm and 830-nm led treatment of photoaging skin. J Drugs Dermatol. 2006; 5:748–53.20. Oshima Y, Coutts RD, Badlani NM, Healey RM, Kubo T, Amiel D. Effect of light-emitting diode (LED) ther-apy on the development of osteoarthritis (OA) in a rabbit model. Biomed Pharmacother. 2011; 65:224–9.
Article21. Chen IC, Chen-Ying S, Chi-Hau F, Hsu-Wei F. Preventative treatment of red light-emitting diode protected osteoarthritis-like chondrocytes from oxidative stress-induced inflammation and promoted matrix gene expression. J Taiwan Inst Chem Eng. 2021; 127:23–31.
Article22. de Morais NC, Barbosa AM, Vale ML, Villaverde AB, de Lima CJ, Cogo JC, et al. Anti-inflammatory effect of low-level laser and light-emitting diode in zymosan-induced arthritis. Photomed Laser Surg. 2010; 28:227–32.
Article23. Jekal SJ, Kwon PS, Kim JK, Lee JH. Effect of 840 ㎚ light-emitting diode (LED) irradiation on monosodium iodoacetate-induced osteoarthritis in rats. J Korean Soc Phys Med. 2014; 9:151–9.24. Alves-Simões M. Rodent models of knee osteoarthritis for pain research. Osteoarthritis Cartilage. 2022; 30:802–14.
Article25. Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, et al. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016; 6:33038.
Article26. Sestakova N, Puzserova A, Kluknavsky M, Bernatova I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol. 2013; 6:126–35.
Article27. Watanabe M, Campbell TM, Reilly K, Uhthoff HK, Laneuville O, Trudel G. Bone replaces unloaded articu-lar cartilage during knee immobilization. A longitudinal study in the rat. Bone. 2021; 142:115694.
Article28. Harro J. Animals, anxiety, and anxiety disorders: how to measure anxiety in rodents and why. Behav Brain Res. 2018; 352:81–93.
Article29. Liu S, Deng Z, Chen K, Jian S, Zhou F, Yang Y, et al. Cartilage tissue engineering: from proinflammatory and anti‑inflammatory cytokines to osteoarthritis treatments (Review). Mol Med Rep. 2022; 25:99.
Article30. Fulga C. Antiinflammatory effect of laser therapy in rheumatoid arthritis. Rom J Intern Med. 1998; 36:273–9.31. Jawad MM, Husein A, Azlina A, Alam MK, Hassan R, Shaari R. Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J Biomed Opt. 2013; 18:128001.32. Ebrahimi H, Darvish F, Alaeddini M, Etemad-Moghadam S. Comparison between the effect of 810 nm and 940 nm diode laser irradiation on histopathological changes in iatrogenic oral ulcers: an animal study. J Dent (Shiraz). 2021; 22:267–72.33. Taradaj J, Halski T, Kucharzewski M, Urbanek T, Halska U, Kucio C. Effect of laser irradiation at different wavelengths (940, 808, and 658 nm) on pressure ulcer healing: results from a clinical study. Evid Based Complement Alternat Med. 2013; 2013:960240.34. Baracho VDS, Chaves MEA, Huebner R, Oliveira MX, Ferreira PHDC, Lucas TC. Phototherapy (cluster multi-diode 630 nm and 940 nm) on the healing of pressure injury: a pilot study. J Vasc Nurs. 2021; 39:67–75.
Article35. Alves AC, Vieira R, Leal-Junior E, dos Santos S, Ligeiro AP, Albertini R, et al. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther. 2013; 15:R116.
Article36. Bellido M, Lugo L, Roman-Blas JA, Castañeda S, Caeiro JR, Dapia S, et al. Subchondral bone microstruc-tural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther. 2010; 12:R152.
Article37. Botter SM, van Osch GJ, Waarsing JH, Day JS, Verhaar JA, Pols HA, et al. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology. 2006; 43:379–88.38. Ma L, Zhao X, Liu Y, Wu J, Yang X, Jin Q. Dihydroartemisinin attenuates osteoarthritis by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone. Int J Mol Med. 2021; 47:04855.
Article39. Chen H, Wang Z, Zhang X, Sun M. Effects of low-intensity pulsed ultrasound on knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2022; 36:1153–69.
Article40. Song BW, Park JH, Kim B, Lee S, Lim S, Kim SW, et al. A combinational therapy of articular cartilage defects: rapid and effective regeneration by using low-intensity focused ultrasound after adipose tissue-derived stem cell transplantation. Tissue Eng Regen Med. 2020; 17:313–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The shear bond strength and adhesive failure pattern in bracket bonding with different light-curing methods
- Topical Photodynamic Therapy for Treatment of Actinic Keratosis Using Light-Emitting Diode (LED) Device
- Laparoscopic fluorescence imaging technique for visualizing biliary structures using sodium fluorescein: the result of a preclinical study in a porcine model
- Transfer printing based manufacturing process for flexible micro-light emitting diode phototherapy devices
- Effect of infection control barrier thickness on light curing units