J Korean Med Sci.
2023 Nov;38(46):e358. 10.3346/jkms.2023.38.e358.
Complications of the Central Nervous System in Pediatric Patients With Common Cold Coronavirus Infection During 2014–2019
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2548540
- DOI: http://doi.org/10.3346/jkms.2023.38.e358
Abstract
- Background
In pediatric patients, the common cold coronavirus (ccCoV) usually causes mild respiratory illness. There are reports of coronavirus causing central nervous system (CNS) infection in experimental animal models. Some immunocompromised patients have also been reported to have fatal CNS infections with ccCoV. The aim of this study was to investigate the clinical characteristics of CNS complications related to ccCoV infection.
Methods
From January 2014 to December 2019, a retrospective analysis was performed of medical records from hospitalized patients under 19 years of age whose ccCoV was detected through polymerase chain reaction in respiratory specimens. The CNS complications were defined as clinically diagnosed seizure, meningitis, encephalopathy, and encephalitis.
Results
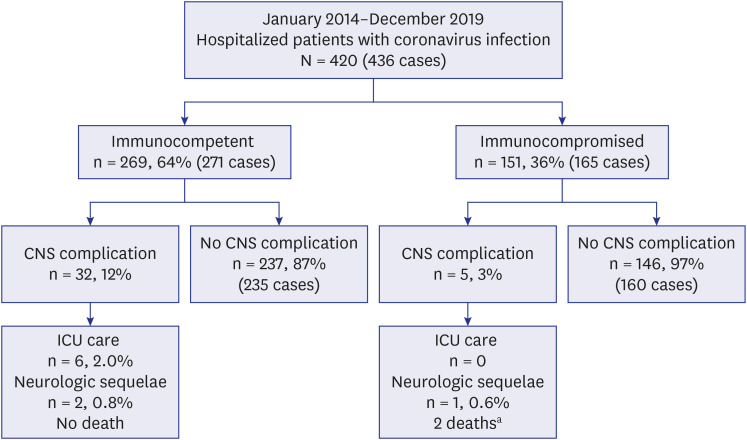
A total of 436 samples from 420 patients were detected as ccCoV. Among the 420 patients, 269 patients were immunocompetent and 151 patients were immunocompromised. The most common type of ccCoV was OC43 (52% in immunocompetent, 37% in immunocompromised). CNS complications were observed in 9.4% (41/436). The most common type of CNS complication was the fever-provoked seizure under pre-existing neurologic disease (42% in immunocompetent and 60% in immunocompromised patients). Among patients with CNS complications, two immunocompetent patients required intensive care unit admission due to encephalitis. Three patients without underlying neurological disease started anti-seizure medications for the first time at this admission. There was no death related to ccCoV infection.
Conclusion
ccCoV infection may cause severe clinical manifestations such as CNS complications or neurologic sequelae, even in previously healthy children.
Figure
Reference
-
1. Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Pediatric Infect Dis Soc. 2019; 8(1):21–28. PMID: 29447395.
Article2. Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures. BMJ. 1965; 1(5448):1467–1470. PMID: 14288084.
Article3. McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967; 57(4):933–940. PMID: 5231356.
Article4. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003; 348(20):1953–1966. PMID: 12690092.
Article5. van der Hoek L, Pyrc K, Berkhout B. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol Rev. 2006; 30(5):760–773. PMID: 16911043.
Article6. Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005; 79(2):884–895. PMID: 15613317.
Article7. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012; 367(19):1814–1820. PMID: 23075143.
Article8. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. PMID: 31978945.
Article9. Ogimi C, Kim YJ, Martin ET, Huh HJ, Chiu CH, Englund JA. What’s new with the old coronaviruses? J Pediatric Infect Dis Soc. 2020; 9(2):210–217. PMID: 32314790.
Article10. Choi YY, Kim YK, Choi EH. Clinical and epidemiological characteristics of common human coronaviruses in children: a single center study, 2015–2019. Pediatr Infect Vaccine. 2021; 28(2):101–109.
Article11. Morfopoulou S, Brown JR, Davies EG, Anderson G, Virasami A, Qasim W, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016; 375(5):497–498. PMID: 27518687.
Article12. Jevšnik M, Steyer A, Pokorn M, Mrvič T, Grosek Š, Strle F, et al. The role of human coronaviruses in children hospitalized for acute bronchiolitis, acute gastroenteritis, and febrile seizures: a 2-year prospective study. PLoS One. 2016; 11(5):e0155555. PMID: 27171141.
Article13. Kasereka MC, Hawkes MT. Neuroinvasive potential of human coronavirus OC43: case report of fatal encephalitis in an immunocompromised host. J Neurovirol. 2021; 27(2):340–344. PMID: 33405204.
Article14. Nilsson A, Edner N, Albert J, Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis (Lond). 2020; 52(6):419–422. PMID: 32067542.
Article15. Seon JY, Jeon WH, Bae SC, Eun BL, Choung JT, Oh IH. Characteristics in pediatric patients with coronavirus disease 2019 in Korea. J Korean Med Sci. 2021; 36(20):e148. PMID: 34032033.
Article16. Choi JH, Choi SH, Yun KW. Risk factors for severe COVID-19 in children: a systematic review and meta-analysis. J Korean Med Sci. 2022; 37(5):e35. PMID: 35132841.
Article17. Lee H, Choi S, Park JY, Jo DS, Choi UY, Lee H, et al. Analysis of critical COVID-19 cases among children in Korea. J Korean Med Sci. 2022; 37(1):e13. PMID: 34981683.
Article18. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020; 19(9):767–783. PMID: 32622375.
Article19. Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience. 2020; 23(12):101839. PMID: 33251489.
Article20. Abdelaziz OS, Waffa Z. Neuropathogenic human coronaviruses: a review. Rev Med Virol. 2020; 30(5):e2118. PMID: 32687681.
Article21. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014; 30(15):2114–2120. PMID: 24695404.
Article22. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012; 19(5):455–477. PMID: 22506599.
Article23. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215(3):403–410. PMID: 2231712.
Article24. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004; 113(1 Pt 1):e73–e76. PMID: 14702500.25. Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016; 59(3):163–169. PMID: 28103598.
Article26. Iijima H, Kubota M, Ogimi C. Change in seizure incidence in febrile children with COVID-19 in the era of omicron variant of concern. J Pediatric Infect Dis Soc. 2022; 11(11):514–517. PMID: 35984115.
Article27. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018; 12:386. PMID: 30416428.
Article28. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019; 12(1):14. PMID: 31861926.
Article29. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181(2):271–280.e8. PMID: 32142651.
Article30. Morgello S. Coronaviruses and the central nervous system. J Neurovirol. 2020; 26(4):459–473. PMID: 32737861.
Article31. Lin JE, Asfour A, Sewell TB, Hooe B, Pryce P, Earley C, et al. Neurological issues in children with COVID-19. Neurosci Lett. 2021; 743:135567. PMID: 33352286.
Article32. Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol. 2006; 35(3):165–172. PMID: 16939854.
Article33. Choi GJ, Park JY, Choi JS, Choi SR, Kim D, Lee JH, et al. Influenza-associated neurologic complications in hospitalized pediatric patients: a multicenter retrospective study in Republic of Korea. Pediatr Infect Dis J. 2021; 40(12):e466–e471. PMID: 34609108.34. Khandaker G, Zurynski Y, Buttery J, Marshall H, Richmond PC, Dale RC, et al. Neurologic complications of influenza A(H1N1)pdm09: surveillance in 6 pediatric hospitals. Neurology. 2012; 79(14):1474–1481. PMID: 22993280.35. Frankl S, Coffin SE, Harrison JB, Swami SK, McGuire JL. Influenza-associated neurologic complications in hospitalized children. J Pediatr. 2021; 239:24–31.e1. PMID: 34293371.
Article36. Antoon JW, Hall M, Howard LM, Herndon A, Freundlich KL, Grijalva CG, et al. COVID-19 and acute neurologic complications in children. Pediatrics. 2022; 150(5):e2022058167. PMID: 35949041.
Article37. Huang YC, Huang SL, Chen SP, Huang YL, Huang CG, Tsao KC, et al. Adenovirus infection associated with central nervous system dysfunction in children. J Clin Virol. 2013; 57(4):300–304. PMID: 23619053.
Article38. Riccò M, Cerviere MP, Corrado S, Ranzieri S, Marchesi F. Respiratory syncytial virus: an uncommon cause of febrile seizures-results from a systematic review and meta-analysis. Pediatr Rep. 2022; 14(4):464–478. PMID: 36412662.
Article39. Goenka A, Michael BD, Ledger E, Hart IJ, Absoud M, Chow G, et al. Neurological manifestations of influenza infection in children and adults: results of a National British Surveillance Study. Clin Infect Dis. 2014; 58(6):775–784. PMID: 24352349.
Article40. Antoon JW, Hall M, Herndon A, Johnson DP, Brown CM, Browning WL, et al. Prevalence, risk factors, and outcomes of influenza-associated neurologic complications in children. J Pediatr. 2021; 239:32–38.e5. PMID: 34216629.
Article41. Kim JA, Chung JI, Yoon PH, Kim DI, Chung TS, Kim EJ, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001; 22(6):1149–1160. PMID: 11415912.42. Hosseini S, Wilk E, Michaelsen-Preusse K, Gerhauser I, Baumgärtner W, Geffers R, et al. Long-term neuroinflammation induced by influenza A virus infection and the impact on hippocampal neuron morphology and function. J Neurosci. 2018; 38(12):3060–3080. PMID: 29487124.
Article43. van Riel D, Leijten LM, Verdijk RM, GeurtsvanKessel C, van der Vries E, van Rossum AM, et al. Evidence for influenza virus CNS invasion along the olfactory route in an immunocompromised infant. J Infect Dis. 2014; 210(3):419–423. PMID: 24550441.
Article44. Chen LW, Teng CK, Tsai YS, Wang JN, Tu YF, Shen CF, et al. Influenza-associated neurological complications during 2014–2017 in Taiwan. Brain Dev. 2018; 40(9):799–806. PMID: 29891404.
Article45. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019; 20(6):341–355. PMID: 30918369.
Article46. Glaser CA, Honarmand S, Anderson LJ, Schnurr DP, Forghani B, Cossen CK, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006; 43(12):1565–1577. PMID: 17109290.
Article47. Peterson CJ, Sarangi A, Bangash F. Neurological sequelae of COVID-19: a review. Egypt J Neurol Psychiat Neurosurg. 2021; 57(1):122.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of pediatric central nervous system infections
- Rotavirus infection-associated central nervous system complications: clinicoradiological features and potential mechanisms
- The value of computerized axial tomography of the brain in children with central nervous system disorders
- Mycoplasma pneumoniae Infection Presented with Multiple Neurological Complications
- Postoperative Central Nervous System Infection