J Korean Med Sci.
2023 Sep;38(38):e301. 10.3346/jkms.2023.38.e301.
Contact Investigations With a Single Tuberculin Skin Test on Infants Exposed to Tuberculosis in a Postpartum Care Center During the Neonatal Period
- Affiliations
-
- 1Department of Pediatrics, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 3Department of Pathology, Kosin University Gospel Hospital, Busan, Korea
- 4Infectious Disease Control Division, Busan Metropolitan City, Busan, Korea
- 5Division of Tuberculosis Prevention and Control, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 6Division of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 7Department of Pediatrics, Pusan National University Children’s Hospital, Pusan National University School of Medicine, Yangsan, Korea
- KMID: 2546221
- DOI: http://doi.org/10.3346/jkms.2023.38.e301
Abstract
- Background
Tuberculosis (TB) exposure in congregate settings related to neonates is a serious medical and social issue. TB exposure happens during the neonatal period, but contact investigations for exposed infants are usually conducted after the neonatal period. Generally, recommendations for screening and managing close contact are different for neonates and children. Thus, there are challenges in contact investigations. We aimed to report contact investigations with a single tuberculin skin test (TST) on infants exposed to infectious TB in a postpartum care center.
Methods
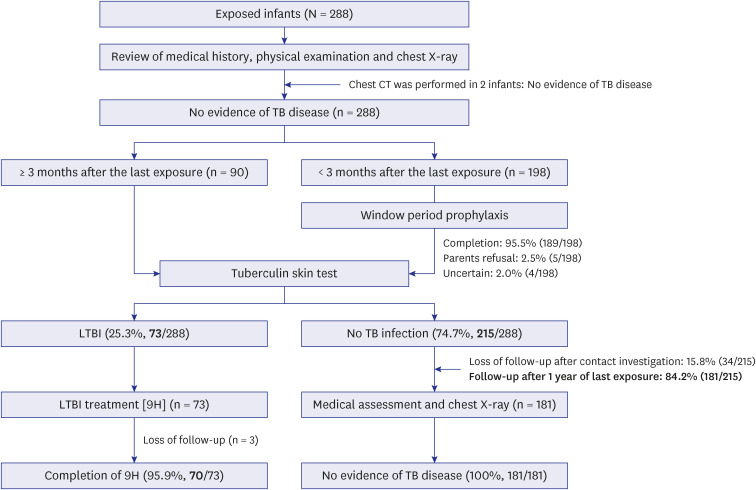
The index case was a healthcare worker with active pulmonary TB: sputum acidfast bacilli smear negative, culture positive, and no cavitary lesion. All exposed infants underwent medical examinations and chest X-ray. After TB disease was ruled out, contacts received window period prophylaxis with isoniazid (INH) until three months after the last exposure. TST was performed only once after completing the prophylaxis.
Results
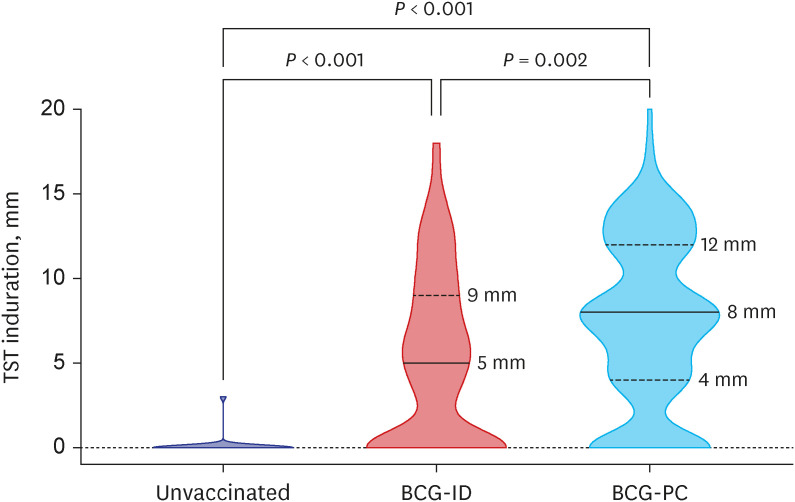
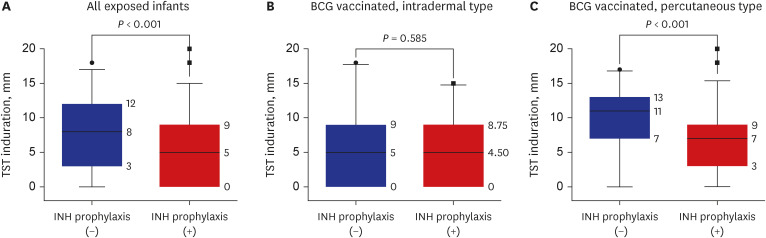
A total of 288 infants were selected as high-priority contacts. At the initial contact investigation, the age of infants ranged from 8 to 114 days. None of these exposed infants had TB disease. The prevalence of latent TB infection (LTBI) was 25.3% (73/288; 95% confidence interval [CI], 20.7–30.7). There were no serious adverse events related to the window period prophylaxis or LTBI treatment with INH. During the 1-year follow-up period, no infants progressed to overt TB disease. The size of TST induration in infants vaccinated with percutaneous Bacillus Calmette-Guérin (BCG) vaccine was significantly larger than that of infants vaccinated with intradermal BCG vaccine (median, 8 mm vs. 5 mm; P = 0.002). In multiple logistic regression analysis, independent factors associated with TST positivity (≥ 10 mm induration) were male (adjusted odds ratio [aOR], 2.98; 95% CI, 1.6–5.64), percutaneous BCG vaccination (aOR, 3.30; 95% CI, 1.75–6.48), TST reading between 60 and 72 hours after injecting purified protein derivative (aOR, 2.87; 95% CI, 1.53–5.49), and INH prophylaxis more than four weeks (aOR, 0.49; 95% CI, 0.25–0.94).
Conclusion
A single TST at three months after the last TB exposure with INH prophylaxis could be used as a main protocol in contact investigations for infants exposed to infectious TB during the neonatal period in congregate settings in Korea.
Figure
Reference
-
1. Korea Disease Control and Prevention Agency. Annual report on the notified tuberculosis in Korea, 2021. Updated 2022. Accessed January 21, 2023. https://npt.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=2770 .2. Go U, Park M, Kim UN, Lee S, Han S, Lee J, et al. Tuberculosis prevention and care in Korea: Evolution of policy and practice. J Clin Tuberc Other Mycobact Dis. 2018; 11:28–36. PMID: 31720389.
Article3. Min J, Kim HW, Stagg HR, Lipman M, Rangaka MX, Myong JP, et al. Latent tuberculosis infection screening and treatment in congregate settings (TB FREE COREA): protocol for a prospective observational study in Korea. BMJ Open. 2020; 10(2):e034098.
Article4. Korea Disease Control and Prevention Agency. Report on tuberculosis epidemiological investigations, 2021. Updated 2022. Accessed January 21, 2023. https://tbzero.kdca.go.kr/tbzero/board/boardView.do?leftMenuId=3¶mMenuId=34&boardSeq=7546&crudType=R .5. Park YJ, Park JA, Han S, Kim J, Park S, Kwon Y, et al. Results of the tuberculosis epidemiological investigation congregated settings, 2021. Public Health Wkly Rep. 2022; 15(28):1997–2016.6. Basu Roy R, Whittaker E, Seddon JA, Kampmann B. Tuberculosis susceptibility and protection in children. Lancet Infect Dis. 2019; 19(3):e96–108. PMID: 30322790.
Article7. Martinez L, Cords O, Horsburgh CR, Andrews JR. Pediatric TB Contact Studies Consortium. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet. 2020; 395(10228):973–984. PMID: 32199484.8. Centers for Disease Control and Prevention (US). Self-study modules on tuberculosis. Module 8: contact investigations for tuberculosis. Updated 2014. Accessed December 28, 2022. https://www.cdc.gov/tb/education/ssmodules/pdfs/modules8-508.pdf .9. National Institute for Health and Care Excellence (UK). Tuberculosis NICE guideline [NG33]. Updated 2019. Accessed January 21, 2023. https://www.nice.org.uk/guidance/ng33 .10. Turnbull L, Bell C, Child F. Tuberculosis (NICE clinical guideline 33). Arch Dis Child Educ Pract Ed. 2017; 102(3):136–142. PMID: 27974357.
Article11. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Disease Control and Prevention Agency. Korean Guidelines for Tuberculosis. 4th ed. Cheongju, Korea: Korea Disease Control and Prevention Agency;2020.12. Canadian Tuberculosis Standards - 8th edition. Can J Respir Crit Care Sleep Med. 2022; 6(Suppl 1):1–255.13. World Health Organization. WHO consolidated guidelines on tuberculosis: module 5: management of tuberculosis in children and adolescents. Updated 2022. Accessed December 28, 2022. https://www.who.int/publications/i/item/9789240046764 .14. Nania JJ, Skinner J, Wilkerson K, Warkentin JV, Thayer V, Swift M, et al. Exposure to pulmonary tuberculosis in a neonatal intensive care unit: unique aspects of contact investigation and management of hospitalized neonates. Infect Control Hosp Epidemiol. 2007; 28(6):661–665. PMID: 17520537.
Article15. Ohno H, Ikegami Y, Kishida K, Yamamoto Y, Ikeda N, Taniguchi T, et al. A contact investigation of the transmission of Mycobacterium tuberculosis from a nurse working in a newborn nursery and maternity ward. J Infect Chemother. 2008; 14(1):66–71. PMID: 18297454.
Article16. Fisher KE, Guaran R, Stack J, Simpson S, Krause W, For KD, et al. Nosocomial pulmonary tuberculosis contact investigation in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2013; 34(7):754–756. PMID: 23739083.
Article17. Ahn JG, Kim DS, Kim KH. Nosocomial exposure to active pulmonary tuberculosis in a neonatal intensive care unit. Am J Infect Control. 2015; 43(12):1292–1295. PMID: 26307044.
Article18. Yangthara B, Wutthigate P, Roongmaitree S, Siripattanapipong P, Lapphra K, Kitsommart R, et al. Nosocomial TB in two neonatal intensive care units at a tertiary care centre: infection risk and outcomes. Int J Tuberc Lung Dis. 2021; 25(7):567–572. PMID: 34183102.
Article19. Casalino M, Jegathesan T, Sgro M, Rea E, Muller M, Campbell DM. Tuberculosis exposure from a healthcare worker to patients in a neonatal intensive care unit (NICU). Can J Infect Dis Med Microbiol. 2022; 2022:2659883. PMID: 35812013.
Article20. Pop R, Kaelin MB, Kuster SP, Sax H, Rampini SK, Zbinden R, et al. Low secondary attack rate after prolonged exposure to sputum smear positive miliary tuberculosis in a neonatal unit. Antimicrob Resist Infect Control. 2022; 11(1):148. PMID: 36471416.
Article21. Rinsky JL, Farmer D, Dixon J, Maillard JM, Young T, Stout J, et al. Notes from the field: contact investigation for an infant with congenital tuberculosis infection - North Carolina, 2016. MMWR Morb Mortal Wkly Rep. 2018; 67(23):670–671. PMID: 29902167.
Article22. Oh CE, Kwon GY, Kwon YH, Lee EJ, Park MS, Kim SH, et al. High tuberculosis transmission rate in children with nursery exposure to undetected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2018; 22(9):1031–1036. PMID: 30092868.
Article23. Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021; 148(6):e2021054663. PMID: 34851422.
Article24. Velasco-Arnaiz E, Soriano-Arandes A, Latorre I, Altet N, Domínguez J, Fortuny C, et al. Performance of tuberculin skin tests and interferon-γ release assays in children younger than 5 years. Pediatr Infect Dis J. 2018; 37(12):1235–1241. PMID: 30408005.
Article25. Bennet R, Nejat S, Eriksson M. Effective tuberculosis contact investigation using interferon-gamma release assays. Pediatr Infect Dis J. 2019; 38(4):e76–e78. PMID: 30882747.
Article26. Chiappini E, Storelli F, Tersigni C, Venturini E, de Martino M, Galli L. QuantiFERON-TB Gold In-Tube test performance in a large pediatric population investigated for suspected tuberculosis infection. Paediatr Respir Rev. 2019; 32:36–47. PMID: 31155511.
Article27. Gaensbauer J, Young J, Harasaki C, Aiona K, Belknap R, Haas MK. Interferon-gamma release assay testing in children younger than 2 years in a US-based health system. Pediatr Infect Dis J. 2020; 39(9):803–807. PMID: 32804462.
Article28. Ho CS, Feng PI, Narita M, Stout JE, Chen M, Pascopella L, et al. Comparison of three tests for latent tuberculosis infection in high-risk people in the USA: an observational cohort study. Lancet Infect Dis. 2022; 22(1):85–96. PMID: 34499863.
Article29. Horwitz O, Bunch-Christensen K. Correlation between tuberculin sensitivity after 2 months and 5 years among BCG vaccinated subjects. Bull World Health Organ. 1972; 47(1):49–58. PMID: 4538905.30. Castro-Rodriguez JA, Mallol J, Andrade R, Muñoz M, Azzini I. Comparison of tuberculin skin test response after three modalities of neonatal BCG vaccination. Trans R Soc Trop Med Hyg. 2007; 101(5):493–496. PMID: 16997338.31. Kemp EB, Belshe RB, Hoft DF. Immune responses stimulated by percutaneous and intradermal bacille Calmette-Guérin. J Infect Dis. 1996; 174(1):113–119. PMID: 8655980.
Article32. Joos TJ, Miller WC, Murdoch DM. Tuberculin reactivity in bacille Calmette-Guérin vaccinated populations: a compilation of international data. Int J Tuberc Lung Dis. 2006; 10(8):883–891. PMID: 16898373.33. Sepulveda RL, Burr C, Ferrer X, Sorensen RU. Booster effect of tuberculin testing in healthy 6-year-old school children vaccinated with Bacillus Calmette-Guérin at birth in Santiago, Chile. Pediatr Infect Dis J. 1988; 7(8):578–581. PMID: 3140208.34. Lifschitz M. The value of the tuberculin skin test as a screening test for tuberculosis among BCG-vaccinated children. Pediatrics. 1965; 36(4):624–627. PMID: 5294418.
Article35. Burl S, Adetifa UJ, Cox M, Touray E, Whittle H, McShane H, et al. The tuberculin skin test (TST) is affected by recent BCG vaccination but not by exposure to non-tuberculosis mycobacteria (NTM) during early life. PLoS One. 2010; 5(8):e12287. PMID: 20808814.
Article36. Seddon JA, Paton J, Nademi Z, Keane D, Williams B, Williams A, et al. The impact of BCG vaccination on tuberculin skin test responses in children is age dependent: evidence to be considered when screening children for tuberculosis infection. Thorax. 2016; 71(10):932–939. PMID: 27335104.
Article37. Kurtz T, Feil AC, Nascimento LS, de Oliveira Abreu P, Scotta MC, Pinto LA. Effect of neonatal bacille Calmette-Guérin on the tuberculin skin test reaction in the first 2 years of life. Int J Tuberc Lung Dis. 2019; 23(3):344–348. PMID: 30871666.
Article38. Aronson JD, Taylor HC, Kirk DL. The effects of isoniazid treatment on the tuberculin reaction and on the healing of BCG vaccine-induced ulcers. Am Rev Tuberc. 1956; 74(1):7–14.39. Narain R, Bagga AS, Naganna K, Mayurnath S. Influence of isoniazid on naturally acquired tuberculin allergy and on induction of allergy by BCG vaccination. Bull World Health Organ. 1970; 43(1):53–64. PMID: 5312322.40. Hsu KH. Tuberculin reaction in children treated with isoniazid. Am J Dis Child. 1983; 137(11):1090–1092. PMID: 6416056.
Article41. Korea Disease Control and Prevention Agency. National tuberculosis management guidelines. Updated 2021. Accessed January 21, 2023. https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&act=view&list_no=711688 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Contact Investigation for Twins With Congenital Tuberculosis in the Neonatal Intensive Care Unit
- Tuberculin skin test in newly employed Health Care Workers
- Outcomes of Child Contact Investigations of Active Pulmonary Tuberculosis Patients: A Single Center Experience from 2012 to 2014
- Tuberculin skin test and measles vaccination
- Prevalence and Risk Factors for Mycobacterium tuberculosis Infection among Contacts of Pulmonary Tuberculosis Patients