J Cerebrovasc Endovasc Neurosurg.
2023 Sep;25(3):322-332. 10.7461/jcen.2022.E2022.07.002.
A rare case of sacral epidural arteriovenous fistula with concomitant occult multiple lumbar epidural arteriovenous fistulas
- Affiliations
-
- 1Department of Neurosurgery, Saiseikai Utsunomiya Hospital, Utsunomiya, Tochigi, Japan
- 2Department of Radiology, Ashikaga Red Cross Hospital, Ashikaga, Tochigi, Japan
- 3Department of Radiology, Saiseikai Utsunomiya Hospital, Utsunomiya, Tochigi, Japan
- KMID: 2546170
- DOI: http://doi.org/10.7461/jcen.2022.E2022.07.002
Abstract
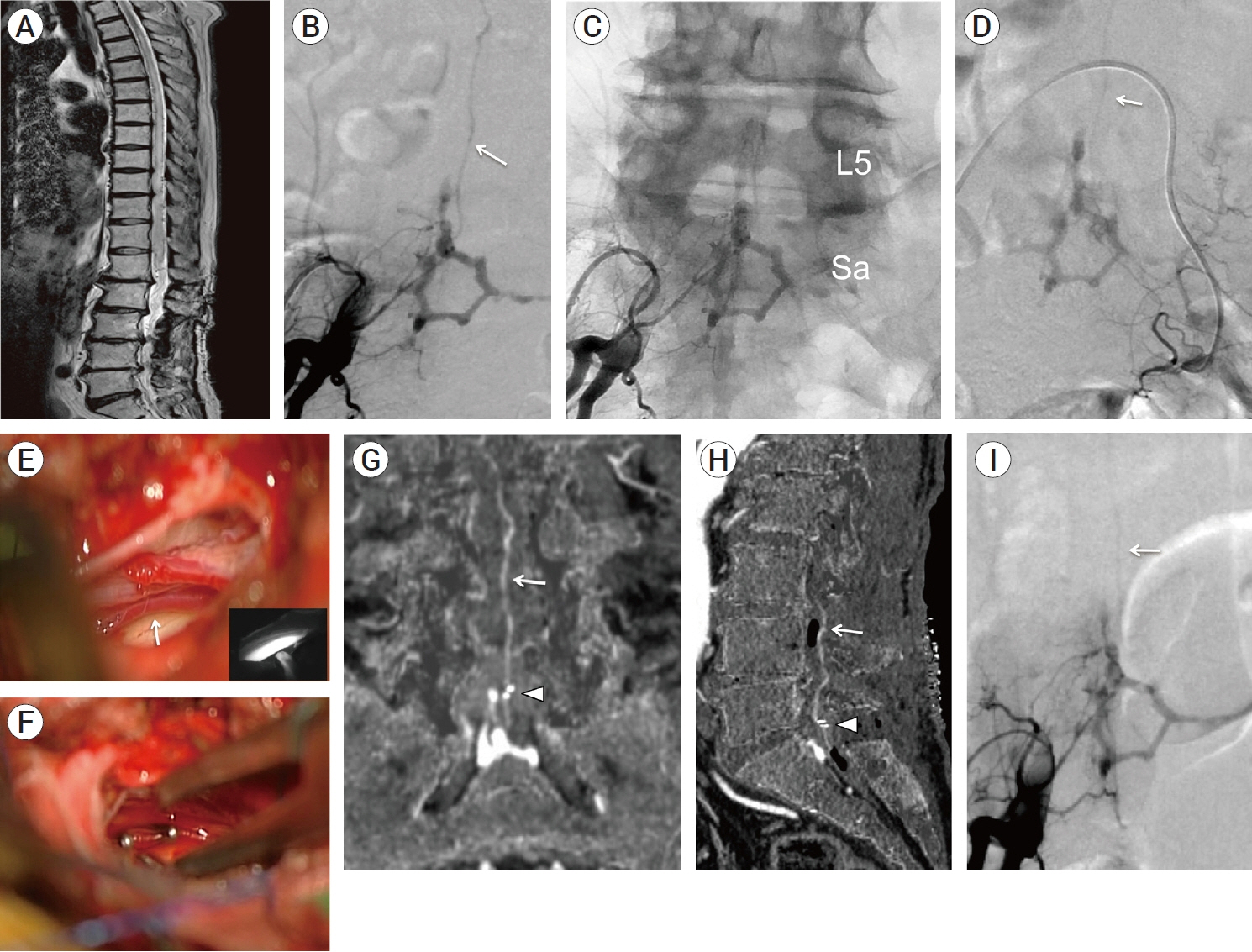
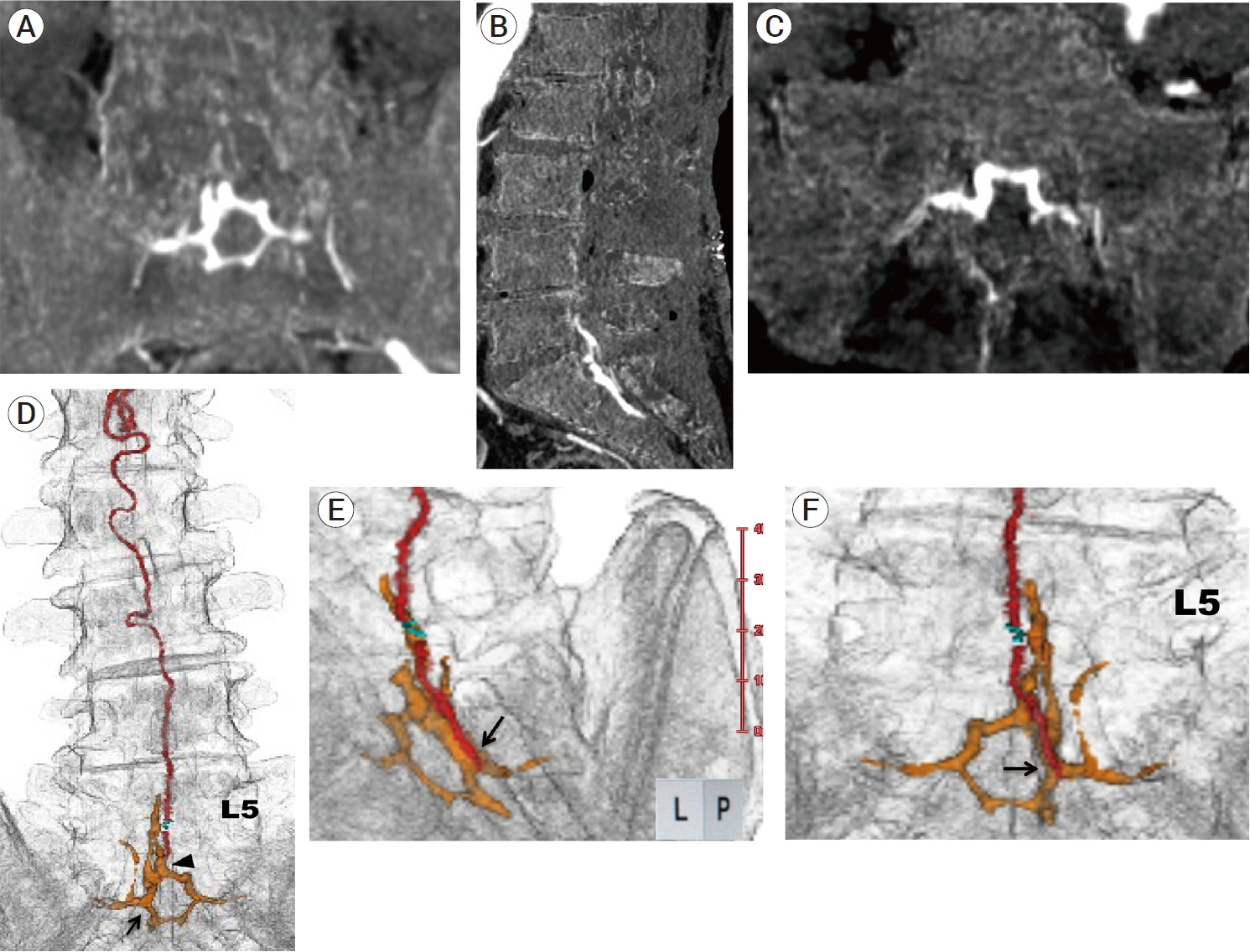
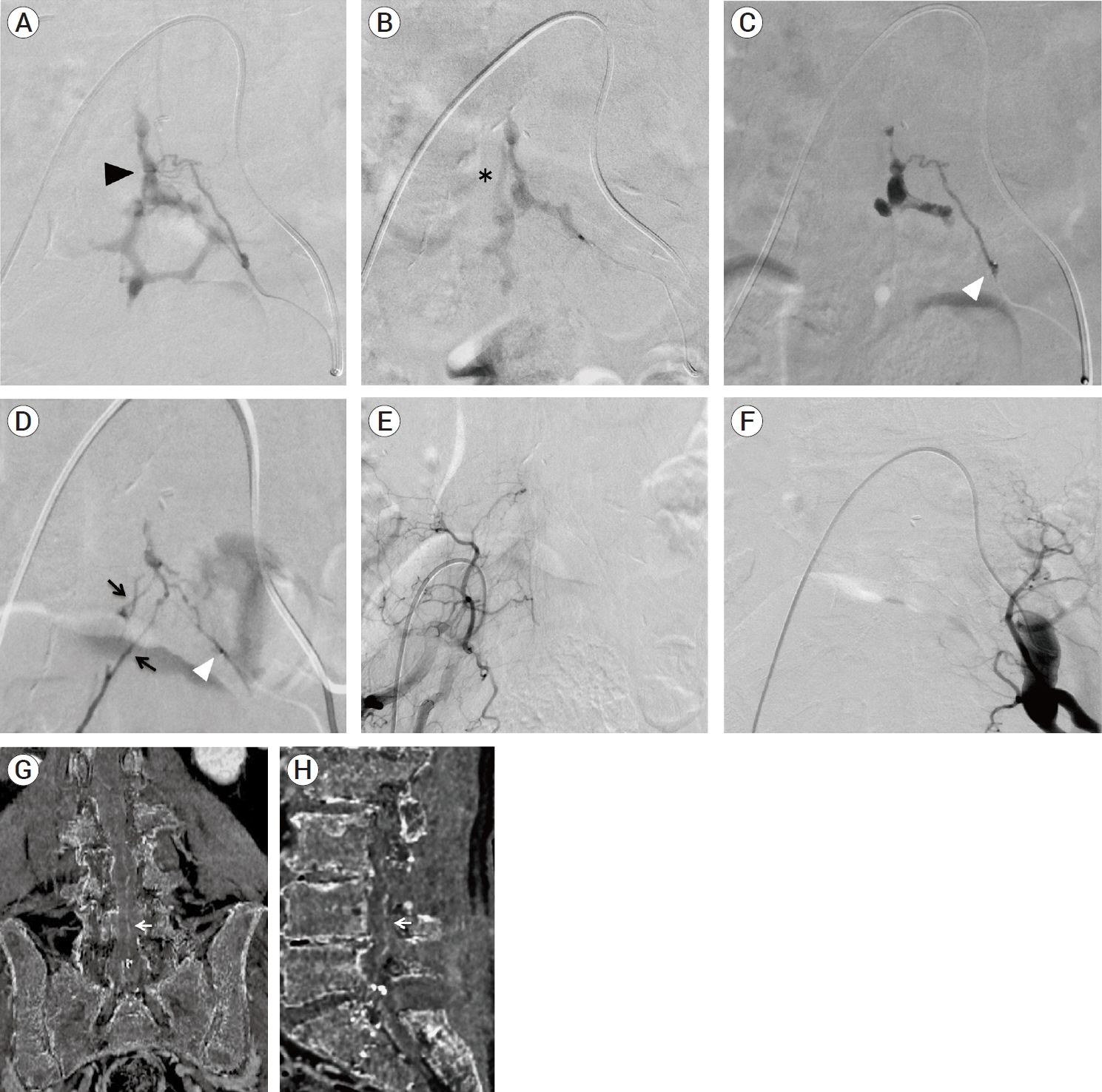
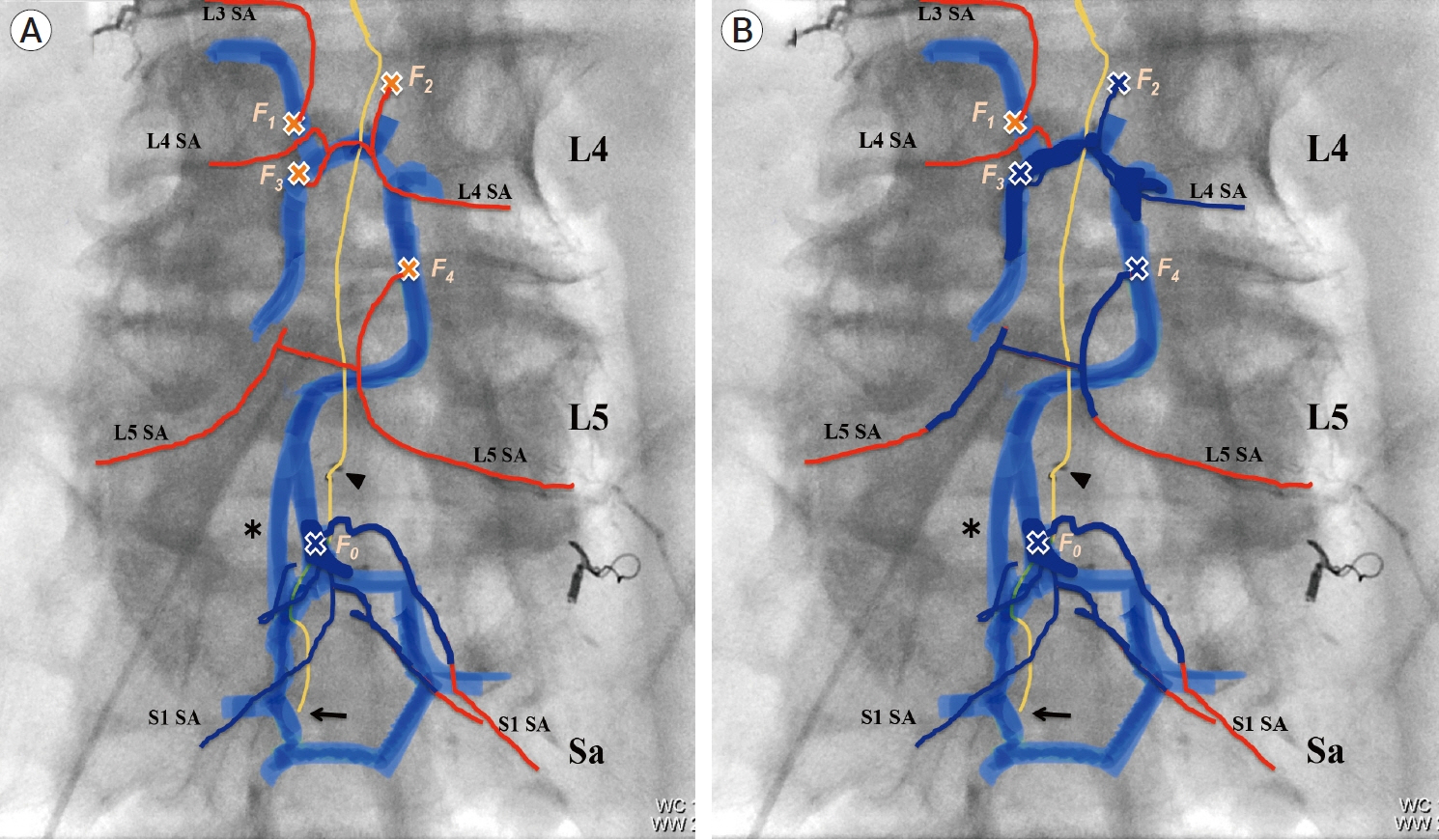
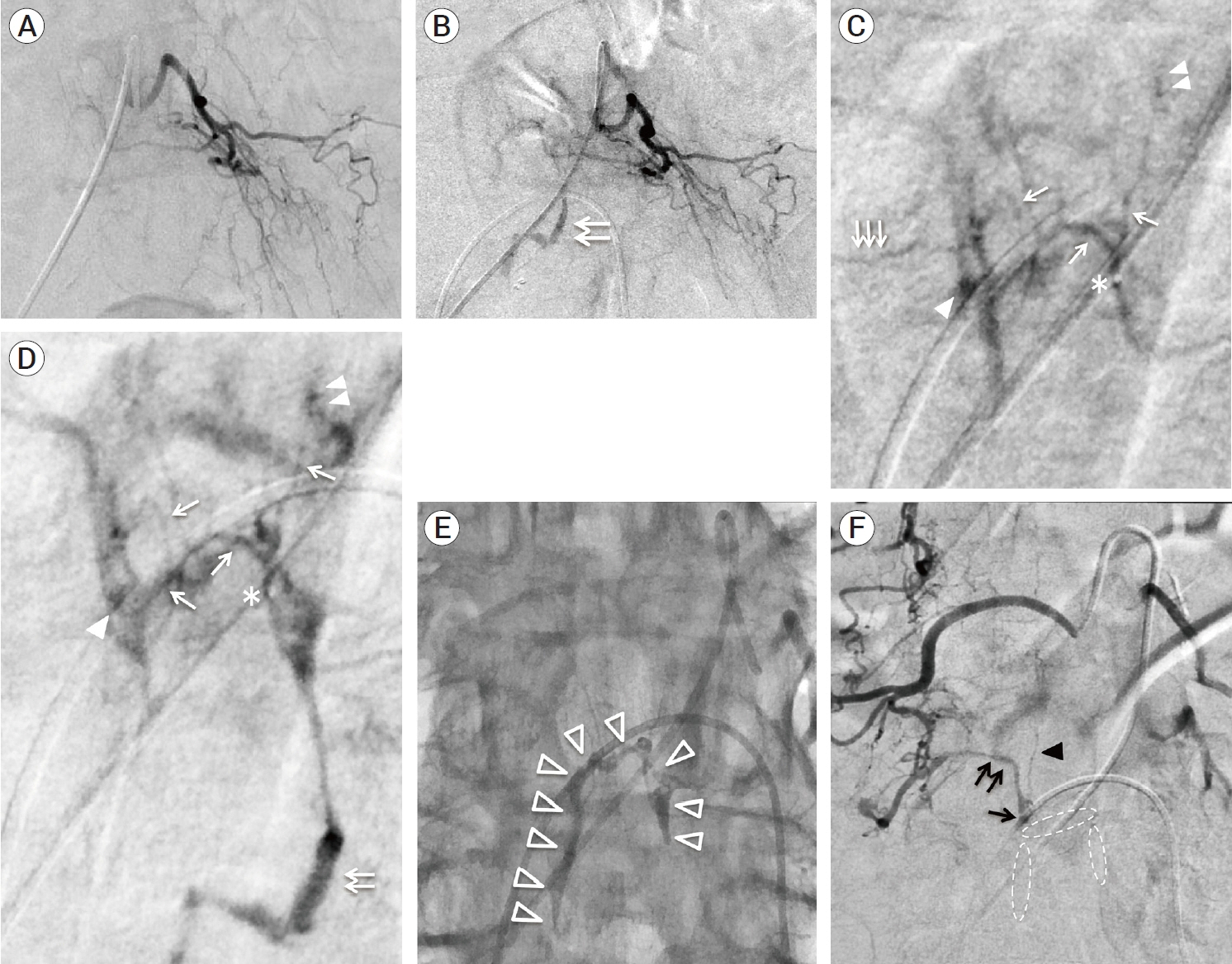
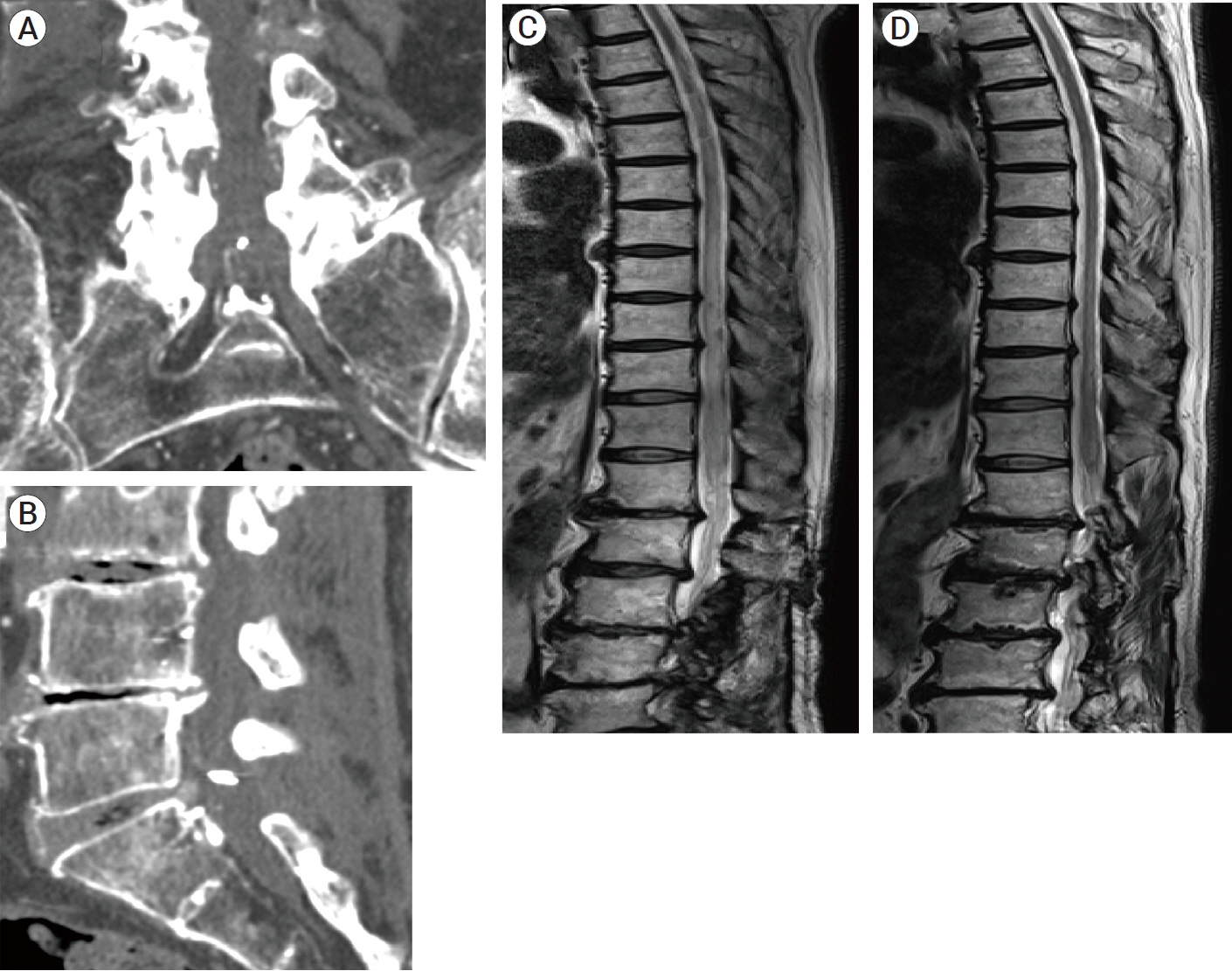
- We describe a rare case of sacral epidural arteriovenous fistulas (edAVFs) with atypical clinical course of treatment. A 78-year-old man with a history of spinal surgery presented progressive gait disturbance and urinary incontinence. Spinal angiography demonstrated a sacral spinal AVF fed by bilateral lateral sacral arteries, draining to the venous pouch with subdural drainage. The first treatment by direct interruption of a subdural drainer was incompletely finished. Postoperative reassessment by 3D imaging analysis led to the diagnosis of sacral edAVF and 3D understanding of its angioarchitecture. The second treatment by transarterial embolization (TAE) resulted in complete occlusion of a sacral edAVF. However, spinal venous congestion didn’t improve, because the recruitment of occult edAVFs at the multiple lumbar levels and complex-shaped sacral ventral epidural venous plexus (VEP) were involved in the remnant of prior subdural drainage. The third treatment was performed by TAE for three occult edAVFs and the VEP compartment connecting between a patent edAVF and subdural drainage, which resulted in complete disappearance of spinal cord edema. Endovascular embolization of VEP compartment connecting to subdural drainage in addition to fistulous occlusion may be one of the treatment options for several edAVFs at the multiple spinal levels.
Keyword
Figure
Reference
-
1. Alvarado AM, Haussen DC, Ebersole K, Nogueira RG, Abraham MG. Embolization of sacral dural arteriovenous fistulas: a case series and literature review. Interv Neurol. 2017; Mar. 6(1-2):73–81.2. Burkhardt J, Safaee MM, Clark AJ, Lawton MT. Sacral epidural arteriovenous fistulas: imitators of spinal dural arteriovenous fistulas with different pathologic anatomy: report of three cases and review of the literature. Acta Neurochir. 2017; Jun. 159(6):1087–92.3. Clarke MJ, Patrick TA, White JB, Cloft HJ, Krauss WE, Lindell EP, et al. Spinal extradural arteriovenous malformations with parenchymal drainage: venous drainage variability and implications in clinical manifestations. Neurosurg Focus. 2009; Jan. 26(1):e5.4. Geibprasert S, Pereira V, Krings T, Jiarakongmun P, Toulgoat F, Pongpech S, et al. Dural arteriovenous shunts: a new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke. 2008; Oct. 39(10):2783–94.5. Groen RJ, Groenewegen HJ, van Alphen HA, Hoogland PV. Morphology of the human internal vertebral venous plexus: a cadaver study after intravenous araldite CY 221 injection. Anat Rec. 1997; Oct. 249(2):285–94.6. Hiramatsu M, Sugiu K, Yasuhara T, Hishikawa T, Nishihiro S, Kidani N, et al. Comparison between spinal dural arteriovenous fistula and spinal epidural arteriovenous fistula. Journal of Neuroendovascular Therapy. 2019; Mar. 13(3):114–9.7. Kiyosue H, Ide S, Uchida S, Kubo T. Dural arteriovenous fistulas: interpretation from the view point of vascular anatomy. No Kekkannai Chiryo. 2020; Jan. 5(1):6–18.8. Kiyosue H, Matsumaru Y, Niimi Y, Takai K, Ishigro T, Hiramatsu M, et al. Angiographic and clinical characteristics of thoracolumbar spinal epidural and dural arteriovenous fistulas. Stroke. 2017; Dec. 48(12):3215–22.9. Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009; Apr. 30(4):639–48.10. Lasjaunias P, Berenstein A, ter Brugge KG. Spinal and Spinal Cord Arteries and Veins. Clinical Vascular Anatomy and Variations. Volume 1, 2nd edition. Berlin, Heidelberg: Springer;2001. p. 73–160.11. Lenck S, Nicholson P, Tymianski R, Hilditch C, Nouet A, Patel K, et al. Spinal and paraspinal arteriovenous lesions. Stroke. 2019; Aug. 50(8):2259–69.12. Patsalides A, Knopman J, Santillan A, Tsiouris AJ, Riina H, Gobin YP. Endovascular treatment of spinal arteriovenous lesions: beyond the dural fistula. AJNR Am J Neuroradiol. 2011; May. 32(5):798–808.13. Ren Y, Liu H, Chen T, You C, Li J. Successful management of sacral dural arteriovenous fistulas: a case series and literature review. World Neurosurg. 2019; Jun. 126:164–70.14. Sasamori T, Hida K, Asano T, Aoyama T, Yamauchi T, Iwasaki M, et al. Sacral dural arteriovenous fistula. Spinal Surgery. 2011; Apr. 25(1):81–3.15. Tadie M, Hemet J, Aaron C, Bianco C, Creissard P, Huard P. Le dispositif protecteur anti-reflux des veines de la moelle. Neurochirurgie. 1979; 25:28–30.16. Takahashi K, Matsumoto Y, Nagata Y, Hashikawa T, Sakai H, Furuta K, et al. The shunt point of the sacral dural arteriovenous fistula: a case report and literature review. World Neurosurg. 2020; Nov. 143:518–26.17. Takai K, Endo T, Yasuhara T, Seki T, Watanabe K, Tanaka Y, et al. Microsurgical versus endovascular treatment of spinal epidural arteriovenous fistulas with intradural venous drainage: a multicenter study of 81 patients. J Neurosurg Spine. 2020; Apr. 24:1–11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endovascular Treatment of Spinal Dural and Epidural Arteriovenous Fistula as Complication of Lumbar Surgery
- Arteriovenous Fistula follwing Lumbar Discectomy
- Spinal Epidural Arteriovenous Fistula Presented with Subdural Hematoma: a Case of Transarterial Embolization Using NBCA
- Acute Paraplegia After Lumbar Steroid Injection in Patients With Spinal Dural Arteriovenous Fistulas: Case Reports
- Congenital Renal Arteriovenous Fistula