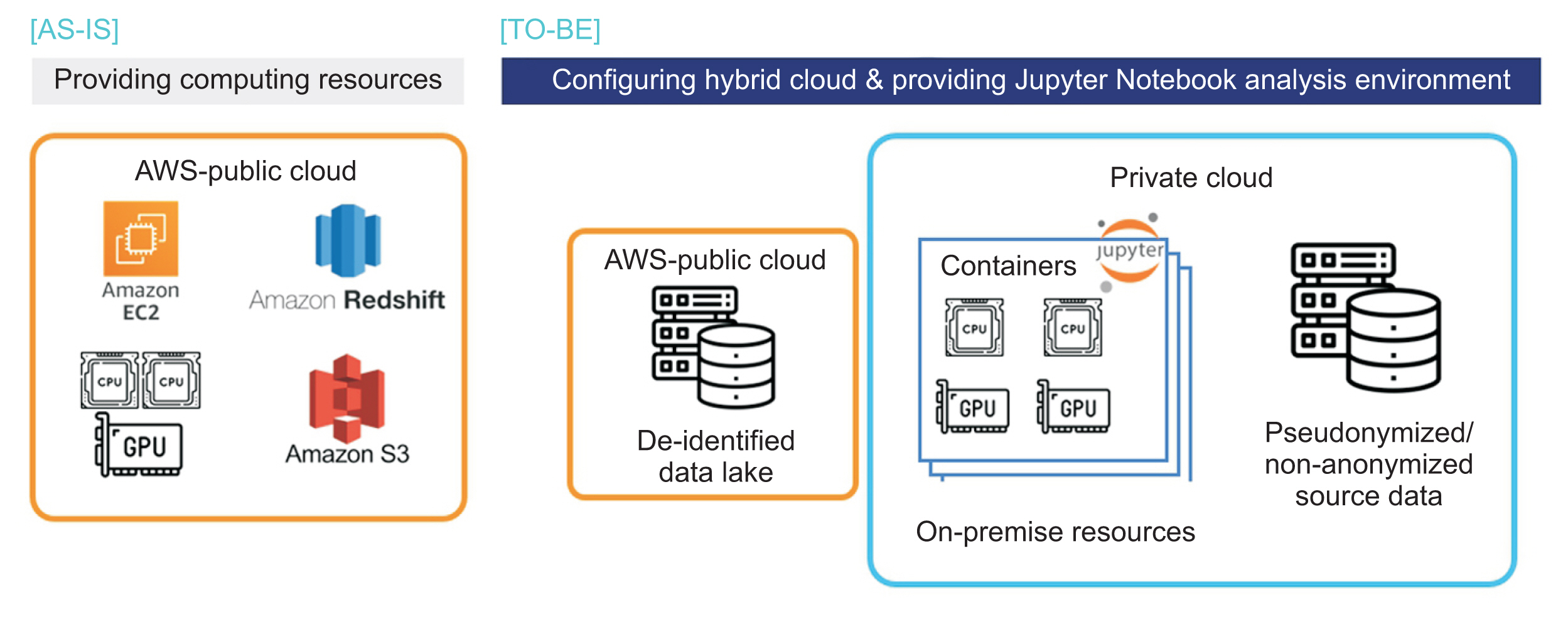
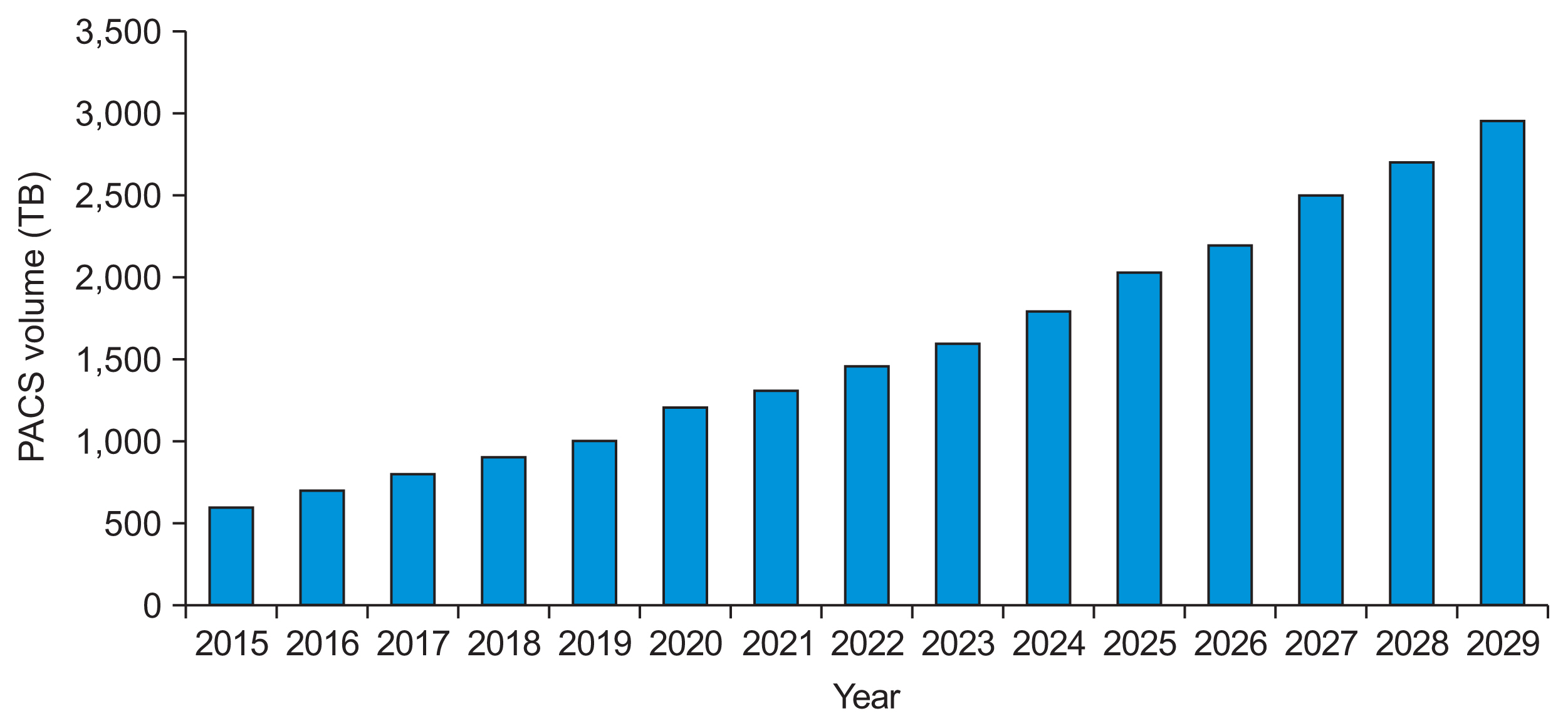
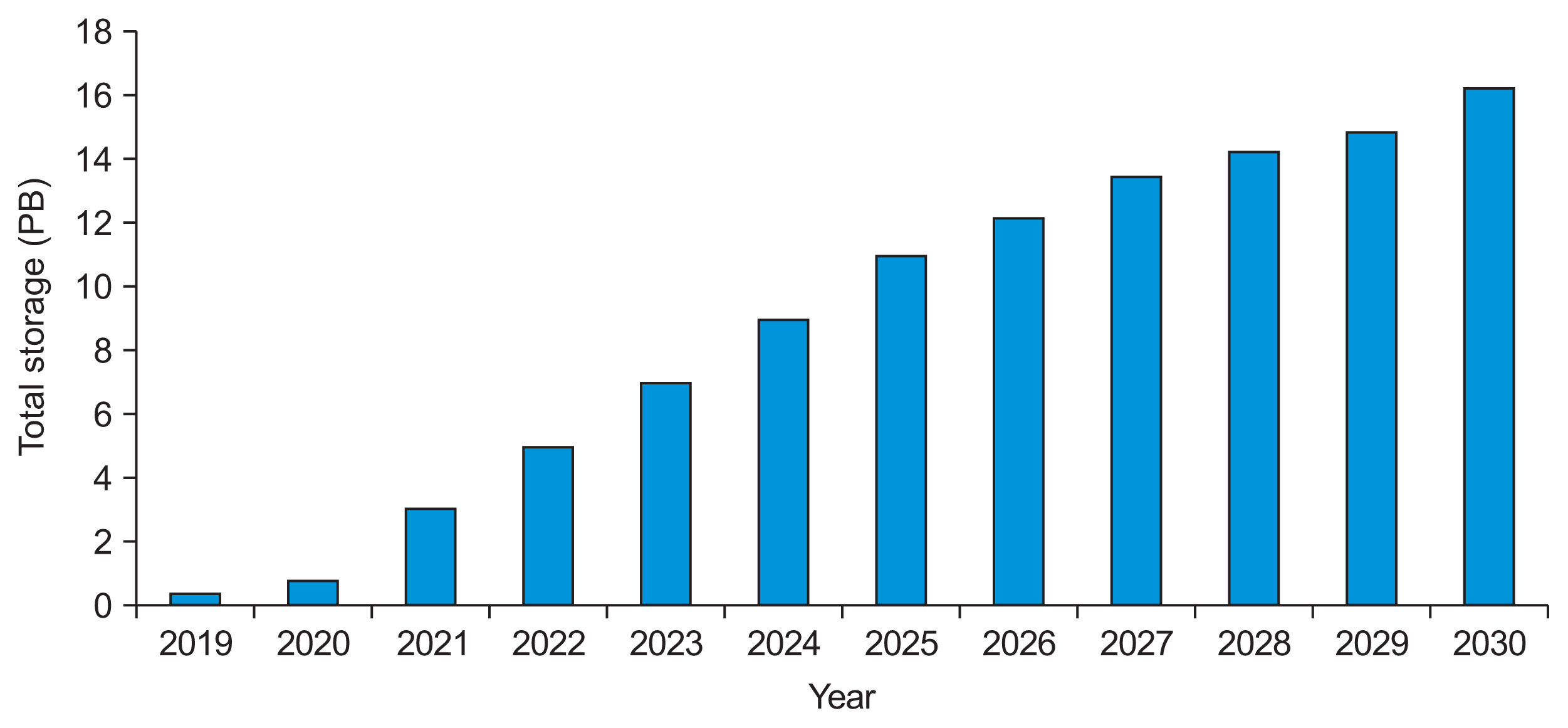
Healthc Inform Res.
2023 Jul;29(3):209-217. 10.4258/hir.2023.29.3.209.
Current Status and Key Issues of Data Management in Tertiary Hospitals: A Case Study of Seoul National University Hospital
- Affiliations
-
- 1Department of Biomedical Engineering, Seoul National University College of Medicine, Seoul, Korea
- 2Interdisciplinary Program for Bioengineering, Graduate School, Seoul National University, Seoul, Korea
- 3Office of Hospital Information, Seoul National University Hospital, Seoul, Korea
- 4Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea
- 5Office of eHealth Research and Businesses, Seoul National University Bundang Hospital, Seongnam, Korea
- 6Department of Nuclear Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 7Information Systems and Technology, Seoul Metropolitan Government-Seoul National University Hospital Boramae Medical Center, Seoul, Korea
- 8Center for Data Science, Biomedical Research Institute, Seoul Metropolitan Government-Seoul National University Hospital Boramae Medical Center, Seoul, Korea
- KMID: 2544872
- DOI: http://doi.org/10.4258/hir.2023.29.3.209
Abstract
Objectives
In the era of the Fourth Industrial Revolution, where an ecosystem is being developed to enhance the quality of healthcare services by applying information and communication technologies, systematic and sustainable data management is essential for medical institutions. In this study, we assessed the data management status and emerging concerns of three medical institutions, while also examining future directions for seamless data management.
Methods
To evaluate the data management status, we examined data types, capacities, infrastructure, backup methods, and related organizations. We also discussed challenges, such as resource and infrastructure issues, problems related to government regulations, and considerations for future data management.
Results
Hospitals are grappling with the increasing data storage space and a shortage of management personnel due to costs and project termination, which necessitates countermeasures and support. Data management regulations on the destruction or maintenance of medical records are needed, and institutional consideration for secondary utilization such as long-term treatment or research is required. Government-level guidelines for facilitating hospital data sharing and mobile patient services should be developed. Additionally, hospital executives at the organizational level need to make efforts to facilitate the clinical validation of artificial intelligence software.
Conclusions
This analysis of the current status and emerging issues of data management reveals potential solutions and sets the stage for future organizational and policy directions. If medical big data is systematically managed, accumulated over time, and strategically monetized, it has the potential to create new value.
Keyword
Figure
Cited by 1 articles
-
Status of MyHealthWay and Suggestions for Widespread Implementation, Emphasizing the Utilization and Practical Use of Personal Medical Data
Taejun Ha, Seonguk Kang, Na Young Yeo, Tae-Hoon Kim, Woo Jin Kim, Byoung-Kee Yi, Jae-Won Jang, Sang Won Park
Healthc Inform Res. 2024;30(2):103-112. doi: 10.4258/hir.2024.30.2.103.
Reference
-
References
1. Korea Health Information Service. Standardization of health and medical information [Internet]. Seoul, Korea: Korea Health Information Service;c2023. [cited at 2023 Aug 2]. Available from: https://www.k-his.or.kr/menu.es?mid=a10203040000.2. Korea Health Information Service. Medical data-driven hospital portal [Internet]. Seoul, Korea: Korea Health Information Service;c2023. [cited at 2023 Aug 2]. Available from: https://www.hins.or.kr/index.es?sid=a2.3. Korea Health Information Service. Personal health record (PHR) [Internet]. Seoul, Korea: Korea Health Information Service;c2023. [cited at 2023 Aug 2]. Available from: https://www.k-his.or.kr/menu.es?mid=a10204000000.4. Cho W. Trial opening of medical MyData, My HealthWay [Internet]. Seoul, Korea: MediGateNews;2022. [cited at 2023 Aug 2]. Available from: https://medigatenews.com/news/2275592704.5. Seoul National University Bundang Hospital. Data review board [Internet]. Seongnam, Korea: Seoul National University Bundang Hospital;c2023. [cited at 2023 Aug 2]. Available from: https://www.snubh.org/member/deliberation.do.6. Korea Law Information Center. Enforcement Rule of the Medical Service Act [Internet]. Sejong, Korea: Ministry of Government Legislation;2015. [updated at 2022 Nov 22, cited at 2023 Aug 2]. Available from: https://law.go.kr/법령/의료법%20시행규칙/제15조.7. Korea Law Information Center. Medical Service Act [Internet]. Sejong, Korea: Ministry of Government Legislation;2002. [updated at 2020 Dec 29, cited at 2023 Aug 2]. Available from: https://glaw.scourt.go.kr/wsjo/lawod/sjo192.do?lawodNm=의료법&jomunNo=21&jomunGajiNo=2.8. Ministry of Food and Drug Safety. Guidelines for approval review of medical devices utilizing big data and artificial intelligence technology [Internet]. Cheongju, Korea: Ministry of Food and Drug Safety;2019. [cited at 2023 Aug 2]. Available from: https://www.mfds.go.kr/brd/m_1060/view.do?seq=14407&srchFr=&srchTo=&srchWord=&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C1004&page=2.9. Ministry of Food and Drug Safety. Guidelines for clinical efficacy evaluation of artificial intelligence-based medical devices [Internet]. Cheongju, Korea: Ministry of Food and Drug Safety;2019. [cited at 2023 Aug 2]. Available from: https://www.mfds.go.kr/brd/m_1060/view.do?seq=14406&srchFr=&srchTo=&srchWord=&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C1004&page=2.10. Ministry of Food and Drug Safety. Guidelines for approval and review of artificial intelligence medical devices [Internet]. Cheongju, Korea: Ministry of Food and Drug Safety;2022. [cited at 2023 Aug 2]. Available from: https://www.mfds.go.kr/brd/m_1060/view.do?seq=15011&srchFr=&srchTo=&srchWord.11. Korea Health Information Service. Healthcare information standardization [Internet]. Seoul, Korea: Korea Health Information Service;c2023. [cited at 2023 Aug 2]. Available from: https://www.k-his.or.kr/menu.es?mid=a20203040000.12. Korea Health Information Service. Innovation of healthcare utilization data (Brief 2022 Vol. 3 [Internet]. Seoul, Korea: Korea Health Information Service;2022. [cited at 2023 Aug 2]. Available from: https://www.k-his.or.kr/board.es?mid=a10309000000&bid=0025.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Key Issues on Long-Term Care Hospitals in Korea
- The Adoption of Electronic Medical Records and Decision Support Systems in Korea
- The Current Status of Hospital Information Systems in Yanbian, China
- Key Issues of Hospital Information Systems Management
- Current status of interprofessional education learning activities in wards provided by tertiary hospitals and secondary general hospitals and barriers