Cancer Res Treat.
2023 Jul;55(3):1001-1010. 10.4143/crt.2022.894.
Clinicopathological Analysis and Treatment of Adult Patients with Inflammatory Myofibroblastic Tumor: A 15-Year Single- Center Study
- Affiliations
-
- 1Department of Head & Neck tumors and Neuroendocrine tumors, Shanghai Medical College, Fudan University, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Department of Breast and Urologic Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 4Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 5Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 6Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China
- KMID: 2544180
- DOI: http://doi.org/10.4143/crt.2022.894
Abstract
- Purpose
Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal malignancy that occurs primarily in children and adolescents. The clinical and pathological features of IMT in adult patients are not well understood.
Materials and Methods
We retrospectively searched for records of adult patients with IMT at Fudan University Shanghai Cancer Center from 2006 to 2021. Clinicopathological data, treatments, and outcomes were collected and analyzed.
Results
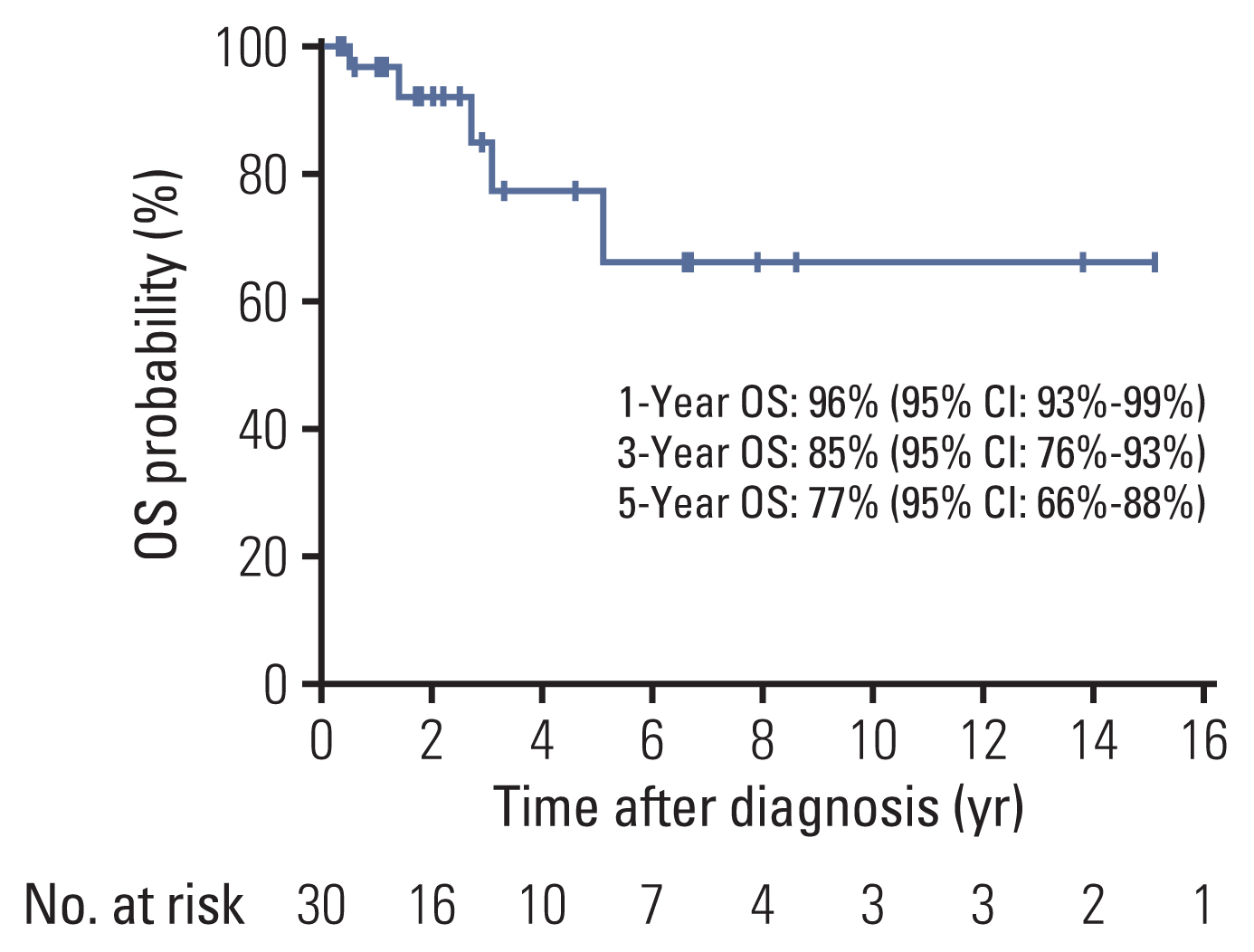
Thirty adult patients with IMT, mostly women (60.0%), were included. The median age of the patients was 38 (21-77). The most common primary site was abdominopelvic region (53.3%), followed by lungs (20.0%). Seven patients had an abdominal epithelioid inflammatory myofibroblast sarcoma (EIMS). The positivity rate of anaplastic lymphoma kinase (ALK) was 81.5% (22/27). Sixteen patients with advanced ALK-positive disease received crizotinib, with an objective response rate (ORR) of 81.3% and a disease control rate of 87.5%. The median progression-free survival was 20.8 months. EIMS was associated with more aggressive behavior; however, the prognosis was similar to that of non-EIMS patients after treatment with an ALK inhibitor. At a median follow-up time of 30 months (95% confidence interval [CI], 13.6 to 46.4), the 5-year overall survival was 77% (95% CI, 66 to 88) in all patients.
Conclusion
Adult IMTs appeared more aggressive, with a higher incidence of recurrence and metastases, and patients with EIMS had more aggressive cases. Treatment with ALK inhibitors resulted in a high ORR and a durable response, which suggested that ALK inhibitors could be used as a first-line treatment option in adult patients with ALK-positive advanced IMT.
Figure
Reference
-
References
1. Nascimento AF, Ruiz R, Hornick JL, Fletcher CD. Calcifying fibrous ‘pseudotumor’: clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol. 2002; 10:189–96.2. Dehner LP. Inflammatory myofibroblastic tumor: the continued definition of one type of so-called inflammatory pseudotumor. Am J Surg Pathol. 2004; 28:1652–4.3. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007; 31:509–20.4. Siminovich M, Galluzzo L, Lopez J, Lubieniecki F, de Davila MT. Inflammatory myofibroblastic tumor of the lung in children: anaplastic lymphoma kinase (ALK) expression and clinico-pathological correlation. Pediatr Dev Pathol. 2012; 15:179–86.
Article5. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995; 19:859–72.
Article6. Lee JC, Wu JM, Liau JY, Huang HY, Lo CY, Jan IS, et al. Cytopathologic features of epithelioid inflammatory myofibroblastic sarcoma with correlation of histopathology, immunohistochemistry, and molecular cytogenetic analysis. Cancer Cytopathol. 2015; 123:495–504.
Article7. Zhou J, Jiang G, Zhang D, Zhang L, Xu J, Li S, et al. Epithelioid inflammatory myofibroblastic sarcoma with recurrence after extensive resection: significant clinicopathologic characteristics of a rare aggressive soft tissue neoplasm. Int J Clin Exp Pathol. 2015; 8:5803–7.8. Chun YS, Wang L, Nascimento AG, Moir CR, Rodeberg DA. Pediatric inflammatory myofibroblastic tumor: anaplastic lymphoma kinase (ALK) expression and prognosis. Pediatr Blood Cancer. 2005; 45:796–801.
Article9. Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999; 59:2776–80.10. Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001; 25:1364–71.11. Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014; 4:889–95.
Article12. Antonescu CR, Suurmeijer AJ, Zhang L, Sung YS, Jungbluth AA, Travis WD, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015; 39:957–67.
Article13. Mahajan P, Casanova M, Ferrari A, Fordham A, Trahair T, Venkatramani R. Inflammatory myofibroblastic tumor: molecular landscape, targeted therapeutics, and remaining challenges. Curr Probl Cancer. 2021; 45:100768.
Article14. Mosse YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children’s Oncology Group Study. J Clin Oncol. 2017; 35:3215–21.
Article15. Fischer M, Moreno L, Ziegler DS, Marshall LV, Zwaan CM, Irwin MS, et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol. 2021; 22:1764–76.
Article16. Pire A, Orbach D, Galmiche L, Berrebi D, Irtan S, Boudjemaa S, et al. Clinical, pathologic, and molecular features of inflammatory myofibroblastic tumors in children and adolescents. Pediatr Blood Cancer. 2022; 69:e29460.
Article17. Casanova M, Brennan B, Alaggio R, Kelsey A, Orbach D, van Noesel MM, et al. Inflammatory myofibroblastic tumor: The experience of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG). Eur J Cancer. 2020; 127:123–9.
Article18. Alaggio R, Cecchetto G, Bisogno G, Gambini C, Calabro ML, Inserra A, et al. Inflammatory myofibroblastic tumors in childhood: a report from the Italian Cooperative Group studies. Cancer. 2010; 116:216–26.19. Webb TR, Slavish J, George RE, Look AT, Xue L, Jiang Q, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009; 9:331–56.
Article20. Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010; 363:1727–33.
Article21. Schoffski P, Kubickova M, Wozniak A, Blay JY, Strauss SJ, Stacchiotti S, et al. Long-term efficacy update of crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumour from EORTC trial 90101 CREATE. Eur J Cancer. 2021; 156:12–23.
Article22. Mansfield AS, Murphy SJ, Harris FR, Robinson SI, Marks RS, Johnson SH, et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann Oncol. 2016; 27:2111–7.
Article23. Carcamo B, Bista R, Wilson H, Reddy P, Pacheco J. Rapid response to lorlatinib in a patient with TFG-ROS1 fusion positive inflammatory myofibroblastic tumor of the chest wall metastatic to the brain and refractory to first and second generation ROS1 inhibitors. J Pediatr Hematol Oncol. 2021; 43:e718–22.
Article24. Haimes JD, Stewart CJ, Kudlow BA, Culver BP, Meng B, Koay E, et al. Uterine inflammatory myofibroblastic tumors frequently harbor ALK fusions with IGFBP5 and THBS1. Am J Surg Pathol. 2017; 41:773–80.
Article25. Marino-Enriquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011; 35:135–44.26. Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol. 2015; 10:106.
Article27. Fang H, Langstraat CL, Visscher DW, Folpe AL, Schoolmeester JK. Epithelioid inflammatory myofibroblastic sarcoma of the ovary with RANB2-ALK fusion: report of a case. Int J Gynecol Pathol. 2018; 37:468–72.28. Fujiya M, Kohgo Y. ALK inhibition for the treatment of refractory epithelioid inflammatory myofibroblastic sarcoma. Intern Med. 2014; 53:2177–8.
Article29. Kimbara S, Takeda K, Fukushima H, Inoue T, Okada H, Shibata Y, et al. A case report of epithelioid inflammatory myofibroblastic sarcoma with RANBP2-ALK fusion gene treated with the ALK inhibitor, crizotinib. Jpn J Clin Oncol. 2014; 44:868–71.
Article30. Kurihara-Hosokawa K, Kawasaki I, Tamai A, Yoshida Y, Yakushiji Y, Ueno H, et al. Epithelioid inflammatory myofibroblastic sarcoma responsive to surgery and an ALK inhibitor in a patient with panhypopituitarism. Intern Med. 2014; 53:2211–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammatory Myofibroblastic Tumor of Nasal Septum after Septoplasty: A Case Report
- Inflammatory Myofibroblastic Tumor of Kidney
- Adult Intussusception Caused by Inflammatory Myofibroblastic Tumor of the Jejunum
- Inflammatory Myofibroblastic Tumor in Posterior Mediastinum
- A Case of Cutaneous Inflammatory Myofibroblastic Tumor