Cancer Res Treat.
2023 Jul;55(3):894-909. 10.4143/crt.2022.1427.
Anti–PD-L1 Antibody and/or 17β-Estradiol Treatment Induces Changes in the Gut Microbiome in MC38 Colon Tumor Model
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine and Liver Research institute, Seoul National University College of Medicine, Seoul, Korea
- 3Laboratory of Immunology, Division of Biotechnology Review and Research-III, Office of Biotechnology Products, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD, USA
- KMID: 2544170
- DOI: http://doi.org/10.4143/crt.2022.1427
Abstract
- Purpose
17β-Estradiol (E2) supplementation suppresses MC38 tumor growth by downregulating the expression of programmed death-ligand 1 (PD-L1). This study aims to figure out the gut microbiota that respond to anti–PD-L1 and/or estrogen treatment in MC38 colon cancer model.
Materials and Methods
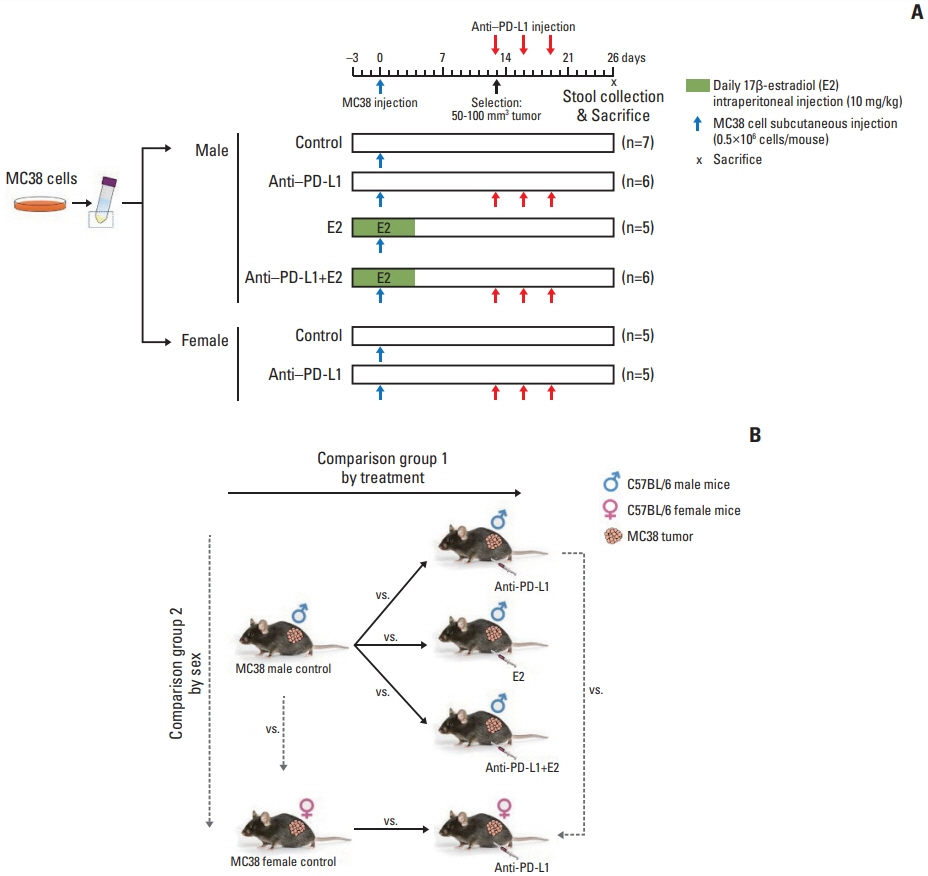
A syngeneic colon tumor model was developed by injection of MC38 cells into C57BL/6 background male and female mice. Three days before MC38 cells injection, E2 was supplemented to male mice daily for 1 week. Male and female mice with MC38 tumors (50-100 mm3) were injected with anti–PD-L1 antibody. Fresh feces were collected 26 days after injection of MC38 cells and 16S rRNA metagenomics sequencing of DNA extracted from feces was used to assess gut microbial composition.
Results
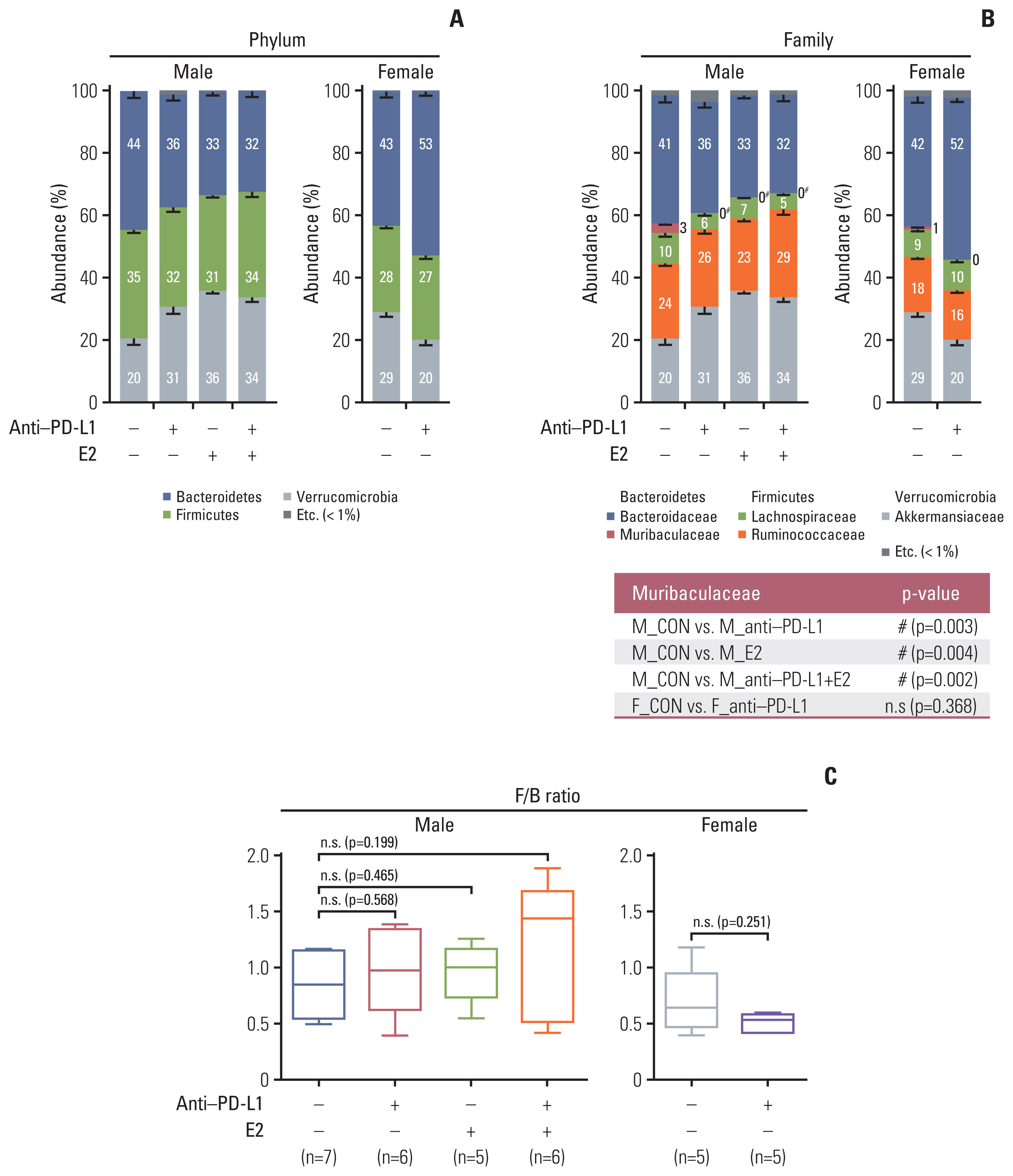
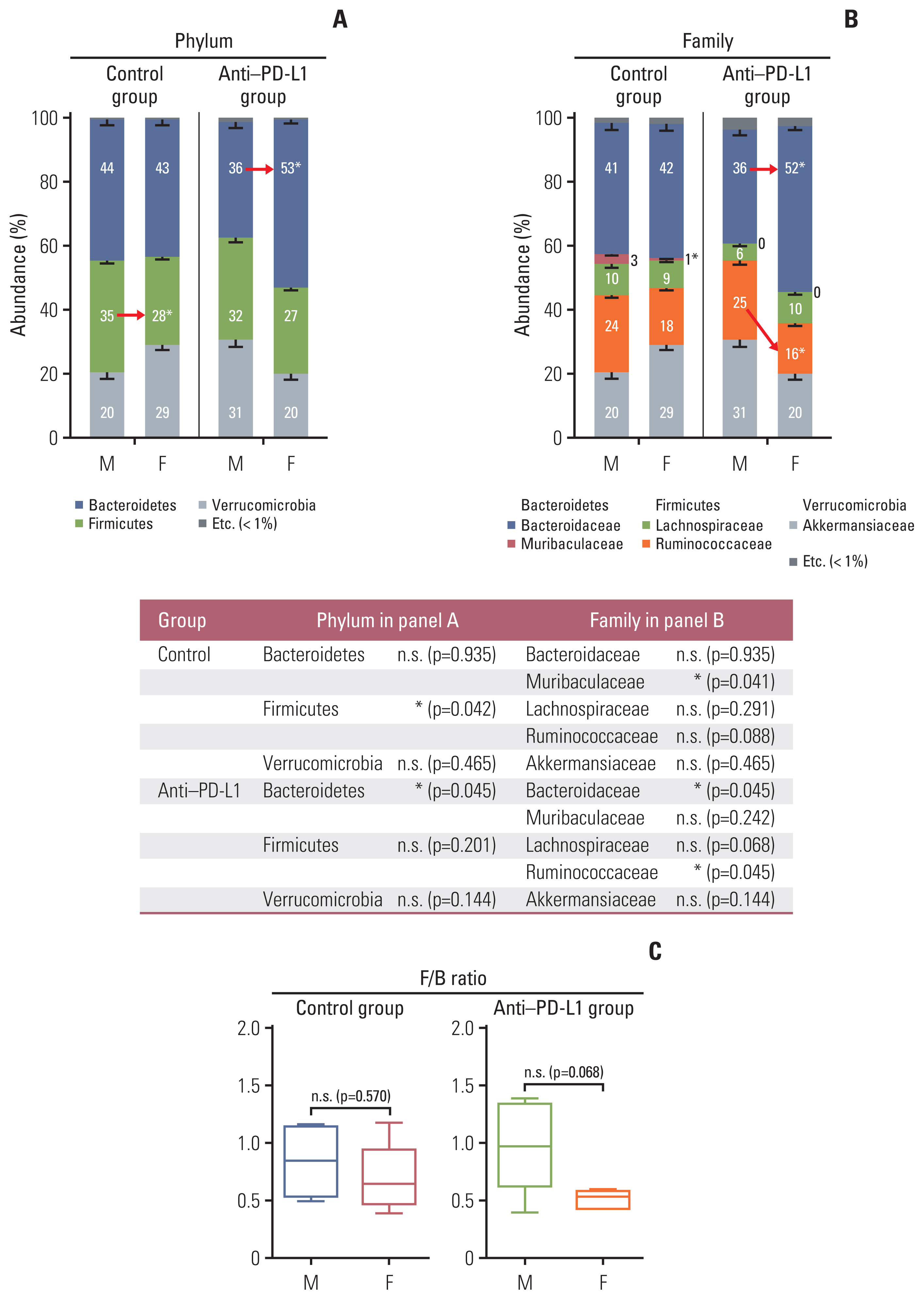
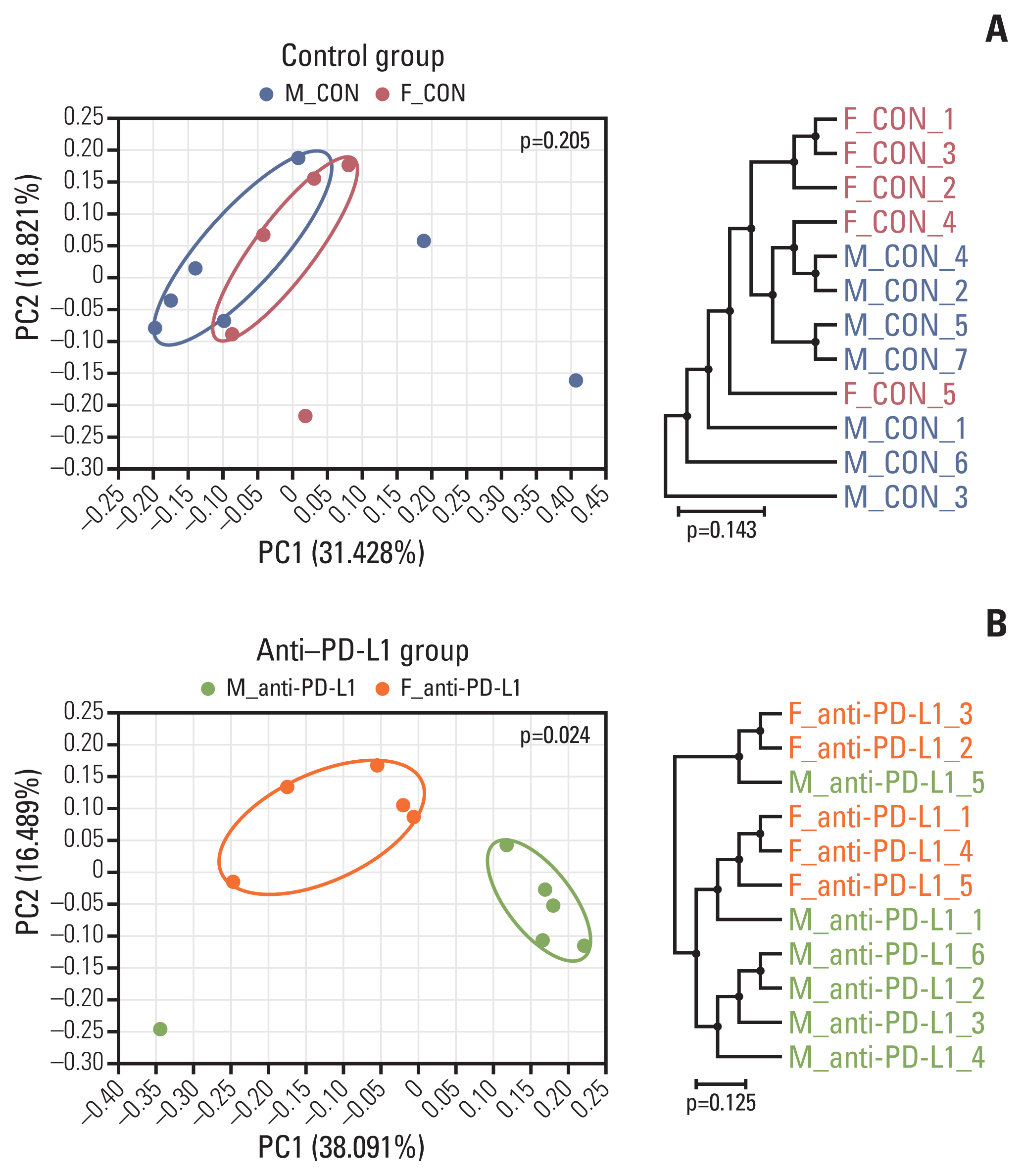
At the taxonomic family level, Muribaculaceae was enriched only in the MC38 male control group. In male mice, linear discriminant analysis effect size analysis at the species level revealed that the four microorganisms were commonly regulated in single and combination treatment with anti–PD-L1 and/or E2; a decrease in PAC001068_g_uc and PAC001070_s (family Muribaculaceae) and increase in PAC001716_s and PAC001785_s (family Ruminococcaceae). Interestingly, in the anti–PD-L1 plus E2 group, a decrease in opportunistic pathogens (Enterobacteriaceae group) and an increase in commensal bacteria (Lactobacillus murinus group and Parabacteroides goldsteinii) were observed. Furthermore, the abundance of Parabacteroides goldsteinii was increased in both males and females in the anti–PD-L1 group.
Conclusion
Our results suggest that gut microbial changes induced by the pretreatment of estrogen before anti–PD-L1 might contribute to treatment of MC38 colon cancer.
Keyword
Figure
Reference
-
References
1. Shanahan F. The colonic microbiota in health and disease. Curr Opin Gastroenterol. 2013; 29:49–54.
Article2. Ternes D, Karta J, Tsenkova M, Wilmes P, Haan S, Letellier E. Microbiome in colorectal cancer: how to get from meta-omics to mechanism? Trends Microbiol. 2020; 28:401–23.
Article3. Chen Y, Chen Y, Zhang J, Cao P, Su W, Deng Y, et al. Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics. 2020; 10:323–39.
Article4. Zhang S, Yang Y, Weng W, Guo B, Cai G, Ma Y, et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J Exp Clin Cancer Res. 2019; 38:14.
Article5. Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015; 21:5167–75.
Article6. Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004; 350:991–1004.
Article7. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017; 103:45–53.
Article8. Chen KL, Madak-Erdogan Z. Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol Metab. 2016; 27:752–5.
Article9. Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016; 7:313–22.
Article10. Song CH, Kim N, Nam RH, Choi SI, Lee HN, Surh YJ. 17beta-Estradiol supplementation changes gut microbiota diversity in intact and colorectal cancer-induced ICR male mice. Sci Rep. 2020; 10:12283.11. Cox-York KA, Sheflin AM, Foster MT, Gentile CL, Kahl A, Koch LG, et al. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol Rep. 2015; 3:e12488.
Article12. Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021; 14:45.
Article13. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020; 11:3801.
Article14. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018; 8:1069–86.15. Son HJ, Sohn SH, Kim N, Lee HN, Lee SM, Nam RH, et al. Effect of estradiol in an azoxymethane/dextran sulfate sodium-treated mouse model of colorectal cancer: implication for sex difference in colorectal cancer development. Cancer Res Treat. 2019; 51:632–48.
Article16. Song CH, Kim N, Nam RH, Choi SI, Jang JY, Kim JW, et al. Combination treatment with 17beta-estradiol and anti-PD-L1 suppresses MC38 tumor growth by reducing PD-L1 expression and enhancing M1 macrophage population in MC38 colon tumor model. Cancer Lett. 2022; 543:215780.17. Hou W, Sampath P, Rojas JJ, Thorne SH. Oncolytic virus-mediated targeting of PGE2 in the tumor alters the immune status and sensitizes established and resistant tumors to immunotherapy. Cancer Cell. 2016; 30:108–19.
Article18. Shi G, Yang Q, Zhang Y, Jiang Q, Lin Y, Yang S, et al. Modulating the tumor microenvironment via oncolytic viruses and CSF-1R inhibition synergistically enhances anti-PD-1 immunotherapy. Mol Ther. 2019; 27:244–60.19. Lee CH, Bae JH, Choe EJ, Park JM, Park SS, Cho HJ, et al. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics. 2022; 12:1971–87.
Article20. Karp NA, Wilson Z, Stalker E, Mooney L, Lazic SE, Zhang B, et al. A multi-batch design to deliver robust estimates of efficacy and reduce animal use - a syngeneic tumour case study. Sci Rep. 2020; 10:6178.
Article21. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014; 30:2114–20.
Article22. Rognes T, Flouri T, Nichols B, Quince C, Mahe F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016; 4:e2584.
Article23. Myers EW, Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988; 4:11–7.
Article24. Wheeler TJ, Eddy SR. nhmmer: DNA homology search with profile HMMs. Bioinformatics. 2013; 29:2487–9.
Article25. Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017; 67:1613–7.
Article26. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCH-IME improves sensitivity and speed of chimera detection. Bioinformatics. 2011; 27:2194–200.
Article27. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011; 12:R60.
Article28. Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019; 68:248–62.
Article29. Lai CH, Lin TL, Huang MZ, Li SW, Wu HY, Chiu YF, et al. Gut commensal Parabacteroides goldsteinii MTS01 alters gut microbiota composition and reduces cholesterol to mitigate Helicobacter pylori-induced pathogenesis. Front Immunol. 2022; 13:916848.
Article30. Ervin SM, Li H, Lim L, Roberts LR, Liang X, Mani S, et al. Gut microbial beta-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. 2019; 294:18586–99.31. Tang C, Kamiya T, Liu Y, Kadoki M, Kakuta S, Oshima K, et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015; 18:183–97.
Article32. Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017; 551:585–9.
Article33. Song CH, Kim N, Nam RH, Choi SI, Yu JE, Nho H, et al. Changes in microbial community composition related to sex and colon cancer by Nrf2 knockout. Front Cell Infect Microbiol. 2021; 11:636808.
Article34. Lee SH, Cho SY, Yoon Y, Park C, Sohn J, Jeong JJ, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021; 6:277–88.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- PD-1: A Negative Regulator of Phagocytosis by Tumour-Associated Macrophages in Colon Cancer
- A Novel Anti-PD-L1 Antibody Exhibits Antitumor Effects on Multiple Myeloma in Murine Models via Antibody-Dependent Cellular Cytotoxicity
- Peripheral blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy
- Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors