Anat Cell Biol.
2023 Jun;56(2):236-251. 10.5115/acb.22.229.
Co-administration of alcohol and combination antiretroviral therapy (cART) in male Sprague Dawley rats: a study on testicular morphology, oxidative and cytokines perturbations
- Affiliations
-
- 1School of Anatomical Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 2Department of Human Anatomy and Physiology, University of Johannesburg, Johannesburg, South Africa
- 3Division of Clinical Anatomy and Biological Anthropology, University of the Cape Town, Cape Town, South Africa
- 4Department of Human Anatomy, Kampala International University, Western Campus, Uganda
- KMID: 2544086
- DOI: http://doi.org/10.5115/acb.22.229
Abstract
- Alcohol consumption alongside combination antiretroviral therapy (cART) has attracted research interest, especially because of increasing male infertility. This study investigated the combined effects of alcohol and cART on testicular morphology, biomarkers of oxidative stress, inflammation, and apoptosis. Rats, weighing 330–370 g, were divided into four groups of six animals each; control, alcohol treated (A), cART, and alcohol plus cART treated (A+cART). Following 90 days treatment period, animals were euthanized, testis extracted, and routinely processed for histology and immunohistochemical analysis. Significantly decreased epithelial area fraction, increased luminal and connective tissue area fractions, and reduction of epithelial height and spermatocyte number, were recorded in the treated groups compared to control. Extensive seminiferous epithelial lesions including widened intercellular space, karyolysis, and sloughing of germinal epithelium were recorded in all the treated groups. Furthermore, upregulation of inducible nitric oxide synthase and 8-hydroxydeoxyguanosine, interleukin-6, and caspase 3 recorded in treated animals, was more significant in A+cART group. Also, the levels of interleukin-1β and tumor necrosis factor-α were more elevated in A and cART treated groups than in A+cART, while MDA was significantly elevated in cART and A+cART treated groups compared to control group. Altogether, the results indicate testicular toxicity of the treatments. It is concluded that consuming alcohol or cART induces oxidative stress, inflammation, and apoptosis in testis of rats, which lead to testicular structural and functional derangements, which are exacerbated when alcohol and cART are consumed concurrently. The result will invaluably assist clinicians in management of reproductive dysfunctions in male HIV/AIDS-alcoholic patients on cART.
Keyword
Figure
Reference
-
References
1. Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. 2019; Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY). 11:10952–91. DOI: 10.18632/aging.102497. PMID: 31790362. PMCID: PMC6932903.
Article2. Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner Selvam MK, Shah R. 2021; Male infertility. Lancet. 397:319–33. DOI: 10.1016/S0140-6736(20)32667-2. PMID: 33308486.
Article3. Simões D, Meireles P, Rocha M, Freitas R, Aguiar A, Barros H. 2021; Knowledge and use of PEP and PrEP among key populations tested in community centers in Portugal. Front Public Health. 9:673959. DOI: 10.3389/fpubh.2021.673959. PMID: 34368050. PMCID: PMC8342856. PMID: a2390f021ce94f848825fb21dcd840ae.
Article4. Ilhan MN, Yapar D. 2020; Alcohol consumption and alcohol policy. Turk J Med Sci. 50:1197–202. DOI: 10.3906/sag-2002-237. PMID: 32421277. PMCID: PMC7491269.
Article5. Vellios NG, Van Walbeek CP. 2017; Self-reported alcohol use and binge drinking in South Africa: evidence from the national income dynamics study, 2014-2015. S Afr Med J. 108:33–9. DOI: 10.7196/SAMJ.2017.v108i1.12615. PMID: 29262976. PMID: a5f8f2e600114530a05ad549107a88a7.
Article6. Trangenstein PJ, Morojele NK, Lombard C, Jernigan DH, Parry CDH. 2018; Heavy drinking and contextual risk factors among adults in South Africa: findings from the International Alcohol Control study. Subst Abuse Treat Prev Policy. 13:43. DOI: 10.1186/s13011-018-0182-1. PMID: 30518429. PMCID: PMC6280515. PMID: 75479f020c3b49eeaf3c5a113e5df811.
Article7. Cornell M, Johnson LF, Wood R, Tanser F, Fox MP, Prozesky H, Schomaker M, Egger M, Davies MA, Boulle A. 2017; Twelve-year mortality in adults initiating antiretroviral therapy in South Africa. J Int AIDS Soc. 20:21902. DOI: 10.7448/IAS.20.1.21902. PMID: 28953328. PMCID: PMC5640314.
Article8. Lippman SA, El Ayadi AM, Grignon JS, Puren A, Liegler T, Venter WDF, Ratlhagana MJ, Morris JL, Naidoo E, Agnew E, Barnhart S, Shade SB. 2019; Improvements in the South African HIV care cascade: findings on 90-90-90 targets from successive population-representative surveys in North West Province. J Int AIDS Soc. 22:e25295. DOI: 10.1002/jia2.25295. PMID: 31190460. PMCID: PMC6562149.
Article9. Mabwe P, Kessy AT, Semali I. 2017; Understanding the magnitude of occupational exposure to human immunodeficiency virus (HIV) and uptake of HIV post-exposure prophylaxis among healthcare workers in a rural district in Tanzania. J Hosp Infect. 96:276–80. DOI: 10.1016/j.jhin.2015.04.024. PMID: 28274607.
Article10. Scott-Sheldon LA, Carey KB, Cunningham K, Johnson BT, Carey MP. 2016; Alcohol use predicts sexual decision-making: a systematic review and meta-analysis of the experimental literature. AIDS Behav. 20(Suppl 1):S19–39. DOI: 10.1007/s10461-015-1108-9. PMID: 26080689. PMCID: PMC4683116.
Article11. Kumar S, Rao PS, Earla R, Kumar A. 2015; Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol. 11:343–55. DOI: 10.1517/17425255.2015.996546. PMID: 25539046. PMCID: PMC4428551.
Article12. Schneider M, Chersich M, Temmerman M, Parry CD. 2016; Addressing the intersection between alcohol consumption and antiretroviral treatment: needs assessment and design of interventions for primary healthcare workers, the Western Cape, South Africa. Global Health. 12:65. DOI: 10.1186/s12992-016-0201-9. PMID: 27784302. PMCID: PMC5080779.
Article13. McCance-Katz EF, Gruber VA, Beatty G, Lum PJ, Rainey PM. 2013; Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. J Addict Med. 7:264–70. DOI: 10.1097/ADM.0b013e318293655a. PMID: 23666322. PMCID: PMC3737351.
Article14. Mondal S, Ghosh P, Biswas D, Roy PK. 2019; Effect of alcohol consumption during antiretroviral therapy on HIV-1 replication: role of Cytochrome P3A4 enzyme. Int J Math Eng Manag Sci. 4:922–35. DOI: 10.33889/IJMEMS.2019.4.4-073. PMID: 25d0266358a9447db169a13a24694354.
Article15. Chander G, Lau B, Moore RD. 2006; Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 43:411–7. DOI: 10.1097/01.qai.0000243121.44659.a4. PMID: 17099312. PMCID: PMC2704473.16. Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, Rodriguez MC, Rabeneck L, Bryant K, Justice AC. 2007; Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 19:459–66. DOI: 10.1080/09540120601095734. PMID: 17453583. PMCID: PMC3460376.
Article17. Kushnir VA, Lewis W. 2011; Human immunodeficiency virus/acquired immunodeficiency syndrome and infertility: emerging problems in the era of highly active antiretrovirals. Fertil Steril. 96:546–53. DOI: 10.1016/j.fertnstert.2011.05.094. PMID: 21722892. PMCID: PMC3165097.
Article18. Ogedengbe OO, Jegede AI, Onanuga IO, Offor U, Peter AI, Akang EN, Naidu ECS, Azu OO. 2018; Adjuvant potential of virgin coconut oil extract on antiretroviral therapy-induced testicular toxicity: an ultrastructural study. Andrologia. 50:e12930. DOI: 10.1111/and.12930. PMID: 29230854.
Article19. Apolikhin OI, Krasnyak SS. 2021; The impact of alcohol on the male reproductive system. Public Health. 1:62–9. DOI: 10.21045/2782-1676-2021-1-2-62-69.
Article20. Duca Y, Aversa A, Condorelli RA, Calogero AE, La Vignera S. 2019; Substance abuse and male hypogonadism. J Clin Med. 8:732. DOI: 10.3390/jcm8050732. PMID: 31121993. PMCID: PMC6571549. PMID: 95bee3aa29c34e11acf61aad123c8807.
Article21. La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. 2013; Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl. 15:221–5. DOI: 10.1038/aja.2012.118. PMID: 23274392. PMCID: PMC3739141.
Article22. Condorelli RA, Calogero AE, Vicari E, La Vignera S. 2015; Chronic consumption of alcohol and sperm parameters: our experience and the main evidences. Andrologia. 47:368–79. DOI: 10.1111/and.12284. PMID: 24766499.
Article23. Oremosu AA, Akang EN. 2015; Impact of alcohol on male reproductive hormones, oxidative stress and semen parameters in Sprague-Dawley rats. Middle East Fertil Soc J. 20:114–8. DOI: 10.1016/j.mefs.2014.07.001. PMID: 2abd059bdc8f40959f73cc24013a8273.
Article24. Van Heertum K, Rossi B. 2017; Alcohol and fertility: how much is too much? Fertil Res Pract. 3:10. DOI: 10.1186/s40738-017-0037-x. PMID: 28702207. PMCID: PMC5504800.
Article25. Oyeyipo IP, Skosana BT, Everson FP, Strijdom H, du Plessis SS. 2018; Highly active antiretroviral therapy alters sperm parameters and testicular antioxidant status in diet-induced obese rats. Toxicol Res. 34:41–8. DOI: 10.5487/TR.2018.34.1.041. PMID: 29372000. PMCID: PMC5776917.
Article26. Savasi V, Parisi F, Oneta M, Laoreti A, Parrilla B, Duca P, Cetin I. 2019; Effects of highly active antiretroviral therapy on semen parameters of a cohort of 770 HIV-1 infected men. PLoS One. 14:e0212194. DOI: 10.1371/journal.pone.0212194. PMID: 30789923. PMCID: PMC6383866. PMID: d9ba924b6d86444593890996fd08b43a.
Article27. Azu OO. 2012; Highly active antiretroviral therapy (HAART) and testicular morphology: current status and a case for a stereologic approach. J Androl. 33:1130–42. DOI: 10.2164/jandrol.112.016758. PMID: 22700761.28. Savasi V, Oneta M, Laoreti A, Parisi F, Parrilla B, Duca P, Cetin I. 2018; Effects of antiretroviral therapy on sperm DNA integrity of HIV-1-infected men. Am J Mens Health. 12:1835–42. DOI: 10.1177/1557988318794282. PMID: 30132391. PMCID: PMC6199444. PMID: 7b652e273f26417a8afc2439cf8d5dff.
Article29. Ogedengbe OO, Naidu ECS, Akang EN, Offor U, Onanuga IO, Peter AI, Jegede AI, Azu OO. 2018; Virgin coconut oil extract mitigates testicular-induced toxicity of alcohol use in antiretroviral therapy. Andrology. 6:616–26. DOI: 10.1111/andr.12490. PMID: 29654715.
Article30. Ogedengbe OO, Naidu ECS, Azu OO. 2018; Antiretroviral therapy and alcohol interactions: X-raying testicular and seminal parameters under the HAART era. Eur J Drug Metab Pharmacokinet. 43:121–35. DOI: 10.1007/s13318-017-0438-6. PMID: 28956285.
Article31. Dutra Gonçalves G, Antunes Vieira N, Rodrigues Vieira H, Dias Valério A, Elóisa Munhoz de Lion Siervo G, Fernanda Felipe Pinheiro P, Eduardo Martinez F, Alessandra Guarnier F, Rampazzo Teixeira G, Scantamburlo Alves Fernandes G. 2017; Role of resistance physical exercise in preventing testicular damage caused by chronic ethanol consumption in UChB rats. Microsc Res Tech. 80:378–86. DOI: 10.1002/jemt.22806. PMID: 27891737.
Article32. Frampton JE, Croom KF. 2006; Efavirenz/emtricitabine/tenofovir disoproxil fumarate: triple combination tablet. Drugs. 66:1501–12. discussion 1513–4. DOI: 10.2165/00003495-200666110-00012. PMID: 16906786.
Article33. Ansa AA, Akpere O, Imasuen JA. 2017; Semen traits, testicular morphometry and histopathology of cadmium-exposed rabbit bucks administered methanolic extract of Phoenix dactylifera fruit. Acta Sci Anim Sci. 39:207–15. DOI: 10.4025/actascianimsci.v39i2.32858. PMID: 8910d82edd03408fbb681a11257d59ec.
Article34. Parhizkar S, Zulkifli SB, Dollah MA. 2014; Testicular morphology of male rats exposed to Phaleria macrocarpa (Mahkota dewa) aqueous extract. Iran J Basic Med Sci. 17:384–90. PMID: 24967068. PMCID: PMC4069844. PMID: c2e411b9a03c421eba236aa034dfd381.35. Kangawa A, Otake M, Enya S, Yoshida T, Shibata M. 2019; Histological changes of the testicular interstitium during postnatal development in microminipigs. Toxicol Pathol. 47:469–82. DOI: 10.1177/0192623319827477. PMID: 30739565.
Article36. Kumar A, Nagar M. 2014; Histomorphometric study of testis in deltamethrin treated albino rats. Toxicol Rep. 1:401–10. DOI: 10.1016/j.toxrep.2014.07.005. PMID: 28962256. PMCID: PMC5598161. PMID: c6477aa8c4b7411a80ae1e87924d9d07.
Article37. Olasile IO, Jegede IA, Ugochukwu O, Ogedengbe OO, Naidu EC, Peter IA, Azu OO. 2018; Histo-morphological and seminal evaluation of testicular parameters in diabetic rats under antiretroviral therapy: interactions with Hypoxis hemerocallidea. Iran J Basic Med Sci. 21:1322–30.38. Qiu LL, Wang X, Zhang XH, Zhang Z, Gu J, Liu L, Wang Y, Wang X, Wang SL. 2013; Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol A. Toxicol Lett. 219:116–24. DOI: 10.1016/j.toxlet.2013.03.011. PMID: 23528252.
Article39. Monteiro JC, da Matta SLP, Predes FS, de Paula TAR. 2012; Testicular morphology of adult Wistar rats treated with Rudgea viburnoides (Cham.) Benth. Leaf infusion. Braz Arch Biol Technol. 55:101–5. DOI: 10.1590/S1516-89132012000100013. PMID: bb92d5d4bea249948b351202e4b9e834.
Article40. Thanh TN, Van PD, Cong TD, Le Minh T, Vu QHN. 2020; Assessment of testis histopathological changes and spermatogenesis in male mice exposed to chronic scrotal heat stress. J Anim Behav Biometeorol. 8:174–80. DOI: 10.31893/jabb.20023.
Article41. Erpek S, Bilgin MD, Dikicioglu E, Karul A. 2007; The effects of low frequency electric field in rat testis. Rev Med Vet. 158:206–12.42. Adaramoye OA, Akanni OO, Adewumi OM, Owumi SE. 2015; Lopinavir/Ritonavir, an antiretroviral drug, lowers sperm quality and induces testicular oxidative damage in rats. Tokai J Exp Clin Med. 40:51–7. PMID: 26150184.43. Fietz D, Pilatz A, Diemer T, Wagenlehner F, Bergmann M, Schuppe HC. 2020; Excessive unilateral proliferation of spermatogonia in a patient with non-obstructive azoospermia - adverse effect of clomiphene citrate pre-treatment? Basic Clin Androl. 30:13. DOI: 10.1186/s12610-020-00111-7. PMID: 32884817. PMCID: PMC7461256. PMID: a589bbaae21f4ccc9c4e1609dbcfb3e6.
Article44. Dosumu OO, Osinubi AAA, Duru FIO. 2014; Alcohol induced testicular damage: can abstinence equal recovery? Middle East Fertil Soc J. 19:221–8. DOI: 10.1016/j.mefs.2014.01.003. PMID: d7e434b690d54ee59bf07e6aaf8031e9.
Article45. Azu OO, Naidu EC, Naidu JS, Masia T, Nzemande NF, Chuturgoon A, Singh S. 2014; Testicular histomorphologic and stereological alterations following short-term treatment with highly active antiretroviral drugs (HAART) in an experimental animal model. Andrology. 2:772–9. DOI: 10.1111/j.2047-2927.2014.00233.x. PMID: 24919589.
Article46. Apa DD, Cayan S, Polat A, Akbay E. 2002; Mast cells and fibrosis on testicular biopsies in male infertility. Arch Androl. 48:337–44. DOI: 10.1080/01485010290099183. PMID: 12230819.
Article47. Yagan N. 2000; Testicular US findings after biopsy. Radiology. 215:768–73. DOI: 10.1148/radiology.215.3.r00jn17768. PMID: 10831698.
Article48. Mayerhofer A. 2013; Human testicular peritubular cells: more than meets the eye. Reproduction. 145:R107–16. DOI: 10.1530/REP-12-0497. PMID: 23431272.
Article49. Wangikar P, Ahmed T, Vangala S. Gupta RC, editor. 2011. Toxicologic pathology of the reproductive system. Reproductive and Developmental Toxicology. Elsevier;London: p. 1003–26. DOI: 10.1016/B978-0-12-382032-7.10076-1.
Article50. Eid N, Ito Y, Otsuki Y. 2013; Anti-apoptotic mechanisms of Sertoli cells against ethanol toxicity. J Alcohol Drug Depend. 1:1000105.
Article51. Pajarinen JT, Karhunen PJ. 1994; Spermatogenic arrest and 'Sertoli cell-only' syndrome--common alcohol-induced disorders of the human testis. Int J Androl. 17:292–9. DOI: 10.1111/j.1365-2605.1994.tb01259.x. PMID: 7744508.
Article52. Trindade AA, Simões AC, Silva RJ, Macedo CS, Spadella CT. 2013; Long term evaluation of morphometric and ultrastructural changes of testes of alloxan-induced diabetic rats. Acta Cir Bras. 28:256–65. DOI: 10.1590/S0102-86502013000400005. PMID: 23568233. PMID: 6977cda41cf541a388591e813566e173.
Article53. Moffit JS, Bryant BH, Hall SJ, Boekelheide K. 2007; Dose-dependent effects of sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol. 35:719–27. DOI: 10.1080/01926230701481931. PMID: 17763286.
Article54. Lie PP, Mruk DD, Lee WM, Cheng CY. 2010; Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 365:1581–92. DOI: 10.1098/rstb.2009.0261. PMID: 20403871. PMCID: PMC2871923.
Article55. Johnson KJ. 2015; Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 4:e979106. DOI: 10.4161/21565562.2014.979106. PMID: 26413393. PMCID: PMC4581046.
Article56. Jelodar G, Khaksar Z, Pourahmadi M. 2009; Endocrine profile and testicular histomorphometry in adult rat offspring of diabetic mothers. J Physiol Sci. 59:377–82. DOI: 10.1007/s12576-009-0045-7. PMID: 19536612.
Article57. Wu Y, Pegoraro AF, Weitz DA, Janmey P, Sun SX. 2022; The correlation between cell and nucleus size is explained by an eukaryotic cell growth model. PLoS Comput Biol. 18:e1009400. DOI: 10.1371/journal.pcbi.1009400. PMID: 35180215. PMCID: PMC8893647. PMID: 750d9852c1cb4d44bf0f0f031d1c2603.
Article58. Zirkin BR, Papadopoulos V. 2018; Leydig cells: formation, function, and regulation. Biol Reprod. 99:101–11. DOI: 10.1093/biolre/ioy059. PMID: 29566165. PMCID: PMC6044347.
Article59. Kumari D, Nair N, Bedwal RS. 2011; Effect of dietary zinc deficiency on testes of Wistar rats: morphometric and cell quantification studies. J Trace Elem Med Biol. 25:47–53. DOI: 10.1016/j.jtemb.2010.11.002. PMID: 21145718.
Article60. Neves BVD, Lorenzini F, Veronez D, Miranda EP, Neves GD, Fraga R. 2017; Numeric and volumetric changes in Leydig cells during aging of rats. Acta Cir Bras. 32:807–15. DOI: 10.1590/s0102-865020170100000002. PMID: 29160367. PMID: 6610a71b4ace45c18d3a3da19317dcc8.
Article61. Akhigbe RE, Hamed MA, Aremu AO. 2021; HAART exacerbates testicular damage and impaired spermatogenesis in anti-Koch-treated rats via dysregulation of lactate transport and glutathione content. Reprod Toxicol. 103:96–107. DOI: 10.1016/j.reprotox.2021.06.007. PMID: 34118364.
Article62. Baydilli N, Akınsal EC, Doğanyiğit Z, Ekmekçioğlu O, Silici S. 2020; The protective role of poplar propolis against alcohol-induced biochemical and histological changes in liver and testes tissues of rats. Erciyes Med J. 42:132–8. DOI: 10.14744/etd.2020.83097. PMID: 4400e04acd2a4716aa2fe7113b3c8328.63. Iftikhar S, Ahmad M, Aslam HM, Saeed T, Yasir A, Nazish GE. 2014; Evaluation of spermatogenesis in prepubertal albino rats with date palm pollen supplement. Afr J Pharm Pharmacol. 8:59–65. DOI: 10.5897/AJPP2013.3662.
Article64. Shokoohi M, Madarek EOS, Khaki A, Shoorei H, Khaki AA, Soltani M, Ainehchi N. 2018; Investigating the effects of onion juice on male fertility factors and pregnancy rate after testicular torsion/detorsion by intrauterine insemination method. Int J Women'. s Health Reprod Sci. 6:499–505. DOI: 10.15296/ijwhr.2018.82.
Article65. Tremellen K. Agarwal A, Aitken R, Alvarez J, editors. 2012. Oxidative stress and male infertility: a clinical perspective. Studies on Men's Health and Fertility. Humana Press;Totowa: p. 325–53. DOI: 10.1007/978-1-61779-776-7_16.
Article66. Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. 2017; The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J Clin Diagn Res. 11:IE01–5. DOI: 10.7860/JCDR/2017/23927.9886. PMID: 28658802. PMCID: PMC5483704. PMID: a61cd15ea4f54cefa5bee06624478a8e.
Article67. Sakr SA, Nooh HZ. 2013; Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats. Anat Cell Biol. 46:122–30. DOI: 10.5115/acb.2013.46.2.122. PMID: 23869259. PMCID: PMC3713276.
Article68. Turner TT, Lysiak JJ. 2008; Oxidative stress: a common factor in testicular dysfunction. J Androl. 29:488–98. DOI: 10.2164/jandrol.108.005132. PMID: 18567643.
Article69. Wu PY, Scarlata E, O'Flaherty C. 2020; Long-term adverse effects of oxidative stress on rat epididymis and spermatozoa. Antioxidants (Basel). 9:170. DOI: 10.3390/antiox9020170. PMID: 32093059. PMCID: PMC7070312. PMID: 0bcd8e3eeb8a4cea87ce5e189f908246.
Article70. Zhao K, Huang Z, Lu H, Zhou J, Wei T. 2010; Induction of inducible nitric oxide synthase increases the production of reactive oxygen species in RAW264.7 macrophages. Biosci Rep. 30:233–41. DOI: 10.1042/BSR20090048. PMID: 19673702.
Article71. Aitken RJ, Roman SD. 2008; Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 1:15–24. DOI: 10.4161/oxim.1.1.6843. PMID: 19794904. PMCID: PMC2715191.
Article72. Sharma R, Agarwal A. Zini A, Agarwal A, editors. 2011. Spermatogenesis: an overview. Sperm Chromatin. Springer;New York: p. 19–44. DOI: 10.1007/978-1-4419-6857-9_2. PMCID: PMC3101747.
Article73. Ikekpeazu JE, Orji OC, Uchendu IK, Ezeanyika LUS. 2020; Mitochondrial and oxidative impacts of short and long-term administration of HAART on HIV patients. Curr Clin Pharmacol. 15:110–24. DOI: 10.2174/1574884714666190905162237. PMID: 31486756. PMCID: PMC7579318.
Article74. Aprioku JS. 2013; Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 14:158–72. PMID: 24551570. PMCID: PMC3911811.75. Dutta S, Sengupta P, Slama P, Roychoudhury S. 2021; Oxidative stress, testicular inflammatory pathways, and male reproduction. Int J Mol Sci. 22:10043. DOI: 10.3390/ijms221810043. PMID: 34576205. PMCID: PMC8471715. PMID: f35700eed32c41b98588927a045e97ea.
Article76. Guerriero G, Trocchia S, Abdel-Gawad FK, Ciarcia G. 2014; Roles of reactive oxygen species in the spermatogenesis regulation. Front Endocrinol (Lausanne). 5:56. DOI: 10.3389/fendo.2014.00056. PMID: 24795696. PMCID: PMC4001055. PMID: 6d008ab0854749afa35cee1eb6fdc956.
Article77. Nna VU, Abu Bakar AB, Ahmad A, Eleazu CO, Mohamed M. 2019; Oxidative stress, NF-κB-mediated inflammation and apoptosis in the testes of streptozotocin-induced diabetic rats: combined protective effects of Malaysian propolis and metformin. Antioxidants (Basel). 8:465. DOI: 10.3390/antiox8100465. PMID: 31600920. PMCID: PMC6826571. PMID: bcf02c1ba2ec49388d12d76acc8a65d1.
Article78. Kolasa A, Marchlewicz M, Kurzawa R, Głabowski W, Trybek G, Wenda-Rózewicka L, Wiszniewska B. 2009; The expression of inducible nitric oxide synthase (iNOS) in the testis and epididymis of rats with a dihydrotestosterone (DHT) deficiency. Cell Mol Biol Lett. 14:511–27. DOI: 10.2478/s11658-009-0019-z. PMID: 19404589. PMCID: PMC6275914.
Article79. Coştur P, Filiz S, Gonca S, Çulha M, Gülecen T, Solakoğlu S, Canberk Y, Çalışkan E. 2012; Êxpression of inducible nitric oxide synthase (iNOS) in the azoospermic human testis. Andrologia. 44(Suppl 1):654–60. DOI: 10.1111/j.1439-0272.2011.01245.x. PMID: 22050043.
Article80. Loveland KL, Klein B, Pueschl D, Indumathy S, Bergmann M, Loveland BE, Hedger MP, Schuppe HC. 2017; Cytokines in male fertility and reproductive pathologies: immunoregulation and beyond. Front Endocrinol (Lausanne). 8:307. DOI: 10.3389/fendo.2017.00307. PMID: 29250030. PMCID: PMC5715375. PMID: 0334cca436b24472b7bc85492ca4e66b.
Article81. Hedger MP, Meinhardt A. 2003; Cytokines and the immune-testicular axis. J Reprod Immunol. 58:1–26. DOI: 10.1016/S0165-0378(02)00060-8. PMID: 12609522.
Article82. Somade OT, Ajayi BO, Safiriyu OA, Oyabunmi OS, Akamo AJ. 2019; Renal and testicular up-regulation of pro-inflammatory chemokines (RANTES and CCL2) and cytokines (TNF-α, IL-1β, IL-6) following acute edible camphor administration is through activation of NF-kB in rats. Toxicol Rep. 6:759–67. DOI: 10.1016/j.toxrep.2019.07.010. PMID: 31413946. PMCID: PMC6687103.
Article83. Kumar S, Jin M, Ande A, Sinha N, Silverstein PS, Kumar A. 2012; Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol. 8:1363–75. DOI: 10.1517/17425255.2012.714366. PMID: 22871069. PMCID: PMC4033313.
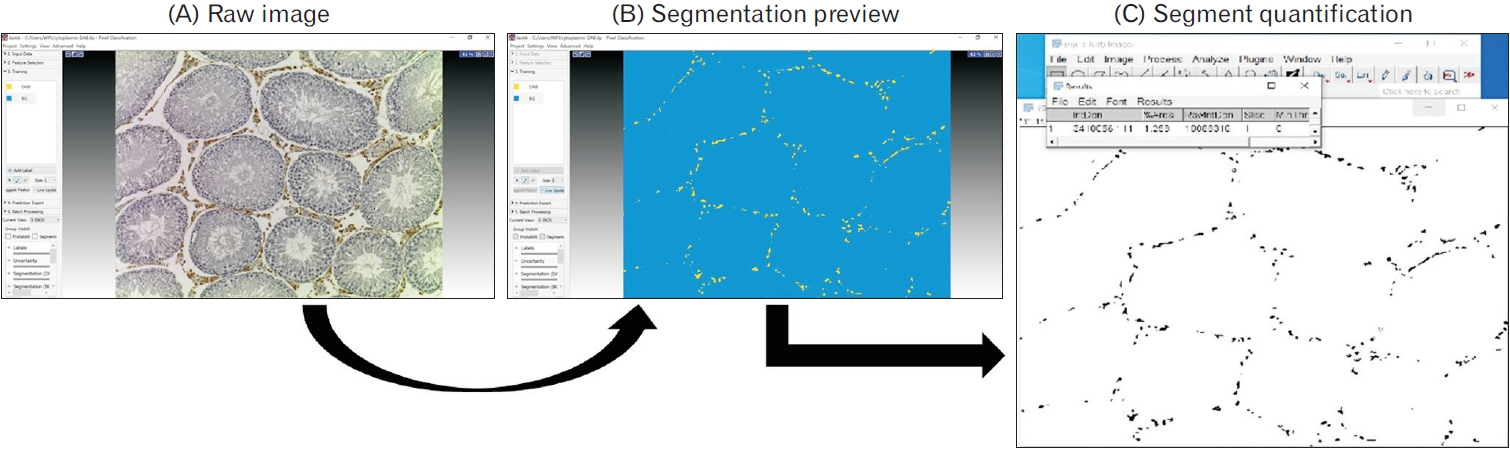
Article84. Berg S, Kutra D, Kroeger T, Straehle CN, Kausler BX, Haubold C, Schiegg M, Ales J, Beier T, Rudy M, Eren K, Cervantes JI, Xu B, Beuttenmueller F, Wolny A, Zhang C, Koethe U, Hamprecht FA, Kreshuk A. 2019; ilastik: interactive machine learning for (bio)image analysis. Nat Methods. 16:1226–32. DOI: 10.1038/s41592-019-0582-9. PMID: 31570887.
Article85. Chim YH, Davies HA, Mason D, Nawaytou O, Field M, Madine J, Akhtar R. 2020; Bicuspid valve aortopathy is associated with distinct patterns of matrix degradation. J Thorac Cardiovasc Surg. 160:e239–57. DOI: 10.1016/j.jtcvs.2019.08.094. PMID: 31679706. PMCID: PMC7674632.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Age-dependent Effect of Metabolism and Testicular Toxicity to di(2-ethylhexyl) Phthalate
- Chemical Orchiectomy Using Absolute Alcohol Injection into Rat Testicles
- Protective Effect of Capsaicin on Contralateral Testis of Rats during Unilateral Testicular Torsion
- The Effect of Experimental Varicocele on Fertility in Adolescent Rats
- Effect of Korean Red Ginseng on Testicular Tissue Injury after Torsion and Detorsion