Ann Pediatr Endocrinol Metab.
2023 Jun;28(2):138-143. 10.6065/apem.2346072.036.
The utilization of basal luteinizing hormone in combination with the basal luteinizing hormone and follicle-stimulating hormone ratio as a diagnostic tool for central precocious puberty in girls
- Affiliations
-
- 1Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 2Division of Pediatric Endocrinology, Department of Pediatrics, Faculty of medicine, Chulalongkorn University, Bangkok, Thailand
- KMID: 2543295
- DOI: http://doi.org/10.6065/apem.2346072.036
Abstract
- Purpose
Intravenous gonadotropin-releasing hormone (IV GnRH) testing is the gold standard for confirming a central precocious puberty (CPP) diagnosis. However, this test is not widely available commercially. Therefore, our study aim was to establish cutoff values for basal gonadotropin level and gonadotrophin responses to a 100-μg subcutaneous IV GnRH test that can distinguish between CPP and premature thelarche (PT) to discover a simple method to detect CPP.
Methods
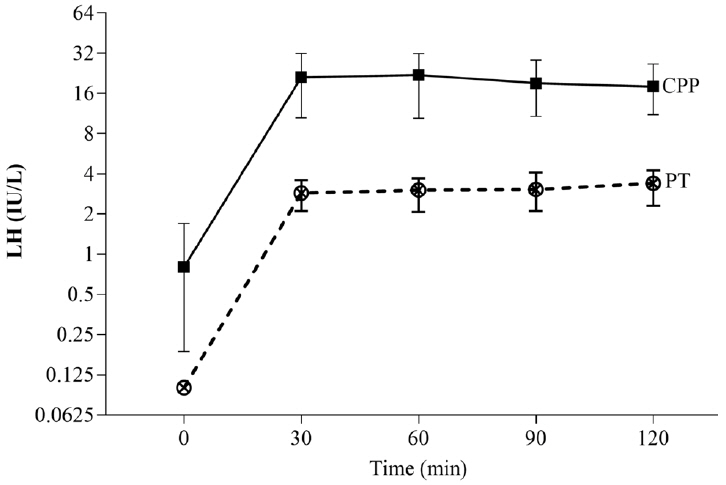
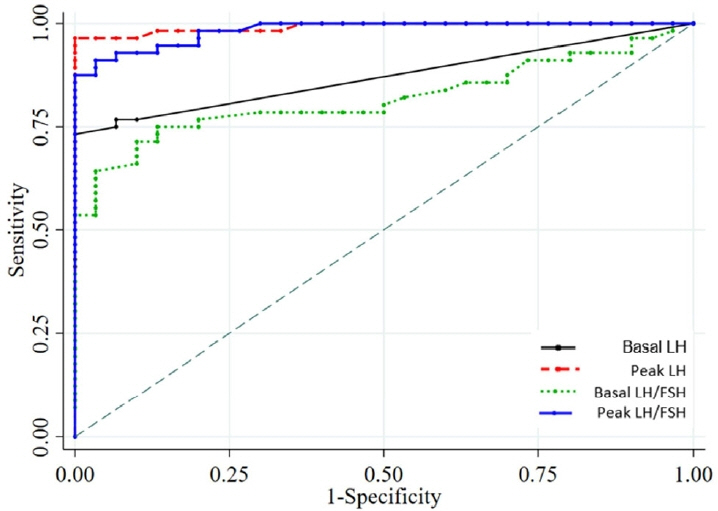
Girls between the ages of 6 and 8 years who attended the pediatric endocrinology outpatient clinic at our tertiary hospital between 2019 and 2022 were included in this study. They were evaluated for breast development, and a subcutaneous 100-μg GnRH test was administered by measuring the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels in blood samples at baseline and then 30, 60, 90, and 120 minutes after injection. CPP is characterized by increased height velocity, advanced bone age, and progression of breast development. The cutoff value for diagnosis of CPP was determined using a receiver operating characteristic (ROC) analysis.
Results
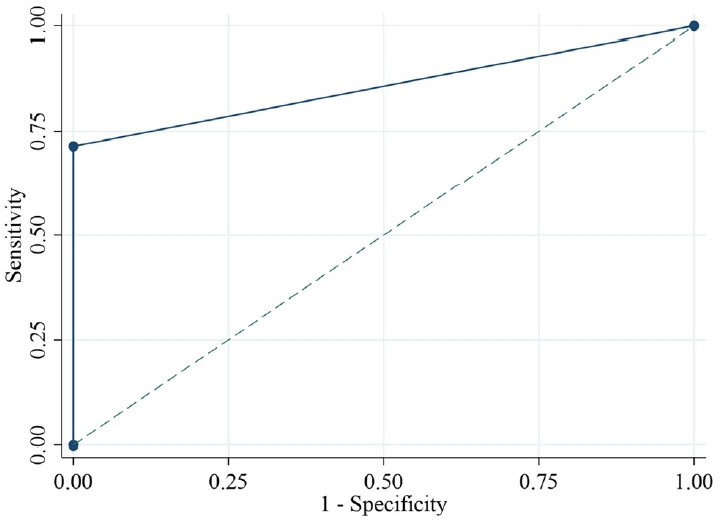
In 86 Thai girls (56 with CPP and 30 with PT), the ROC analysis showed 71.4% and 100% sensitivity and specificity, respectively, for basal LH (cutoff ≥ 0.2 IU/L) plus the basal LH/FSH ratio (cutoff ≥ 0.1). The optimal cutoff values for peak LH (cutoff ≥ 7 IU/L) demonstrated a sensitivity of 94.6% and a specificity of 100%, whereas the LH value at 30 and 60 minutes after injection (cutoff ≥ 6 IU/L) demonstrated sensitivities of 92.9% and 94.6% and a specificity of 100%, respectively
Conclusion
Combining the basal LH (cutoff: 0.2 IU/L) and the basal LH/FSH ratio (cutoff: 0.1) can easily and cost-effectively diagnose CPP in a girl in breast Tanner stage II.
Keyword
Figure
Reference
-
References
1. Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016; 4:254–64.2. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003; 24:668–93.3. Kota AS, Ejaz S. Precocious puberty [Internet]. Treasure Island (FL): StatPearls Publishing;2023. Jan-. [updated 4 July 2022; cited 2022 20 December]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544313.4. Chen M, Eugster EA. Central precocious puberty: update on diagnosis and treatment. Paediatr Drugs. 2015; 17:273–81.5. Fudge E. Precocious puberty. In : Ajjan R, Orme SM, editors. Endocrinology and diabetes: case studies, questions and commentaries. New York: Springer;2022. p. 185–96.6. Yang H, Luo S, Liang X, Lin Q, Cheng T, Zeng L, et al. The association between family impact and health-related quality of life of children with idiopathic central precocious puberty in Chongqing, China. Health Qual Life Outcomes. 2021; 19:171.7. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR MR; ESPE-LWPES GnRH Analogs Consensus Conference Group, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009; 123:e752–62.8. Jung MK, Song KC, Kwon AR, Chae HW, Kim DH, Kim HS. Adult height in girls with central precocious puberty treated with gonadotropin-releasing hormone agonist with or without growth hormone. Ann Pediatr Endocrinol Metab. 2014; 19:214–9.9. Kletter GB, Klein KO, Wong YY. A pediatrician's guide to central precocious puberty. Clin Pediatr (Phila). 2015; 54:414–24.10. Khokhar A, Mojica A. Premature thelarche. Pediatr Ann. 2018; 47:12–5.11. Eugster EA. Update on precocious puberty in girls. J Pediatr Adolesc Gynecol. 2019; 32:455–9.12. Wankanit S, Mahachoklertwattana P, Pattanaprateep O, Poomthavorn P. Basal serum luteinising hormone cut-off, and its utility and cost-effectiveness for aiding the diagnosis of the onset of puberty in girls with early stages of breast development. Clin Endocrinol (Oxf). 2020; 92:46–54.13. Poomthavorn P, Khlairit P, Mahachoklertwattana P. Subcutaneous gonadotropin-releasing hormone agonist (triptorelin) test for diagnosing precocious puberty. Horm Res. 2009; 72:114–9.14. Freire AV, Escobar ME, Gryngarten MG, Arcari AJ, Ballerini MG, Bergada I, et al. High diagnostic accuracy of subcutaneous Triptorelin test compared with GnRH test for diagnosing central precocious puberty in girls. Clin Endocrinol (Oxf). 2013; 78:398–404.15. Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, et al. Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr. 2019; 91:357–72.16. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Redwood City (CA): Stanford University Press;1959.17. Borges MF, Pacheco KD, Oliveira AA, Rita CVC, Pacheco KD, Resende EAM, et al. Premature thelarche clinical and laboratorial. Endocrinol Metab. 2008; 52:93–100.18. Lee PA. laboratory monitoring of children with precocious puberty. Pediatr Adolesc Med. 1994; 148:369–76.19. Parker KL. Depot leuprolide acetate dosage for sexual precocity. Endocrinol Metab. 1991; 73:50–2.20. Neely EK, Hintz RL, Wilson DM, Lee PA, Gautier T, Argente J, et al. Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr. 1995; 127:40–6.21. Neely EK, Wilson DM, Lee PA, Stene M, Hintz RL. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J Pediatr. 1995; 127:47–52.22. Houk CP, Kunselman AR, Lee PA. Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics. 2009; 123:1059–63.23. Harrington J, Palmert MR, Hamilton J. Use of local data to enhance uptake of published recommendations: an example from the diagnostic evaluation of precocious puberty. Arch Dis Child. 2014; 99:15–20.24. Resende EA, Lara BH, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immuno-chemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab. 2007; 92:1424–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influence of Body Mass Index on Luteinizing Hormone Levels after Gonadotropin-Releasing Hormone Stimulation in Girls with Precocious and Advanced Puberty
- The Utility of Basal Serum Luteinizing Hormone Levels for Screening Central Precocious Puberty in Girls
- Basal luteinizing hormone and follicular stimulating hormone: is it sufficient for the diagnosis of precocious puberty in girls?
- A case of idiopathic precocious puberty treated with a luteinizing hormone relaeasing hormone analog
- Diurnal variation of gonadotropin levels in girls with early stages of puberty