J Korean Med Sci.
2023 Jun;38(22):e173. 10.3346/jkms.2023.38.e173.
Population Pharmacokinetic− Pharmacodynamic Modeling of Carvedilol to Evaluate the Effect of Cytochrome P450 2D6 Genotype on the Heart Rate Reduction
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine, Seoul, Korea
- 2Kidney Research Institute, Seoul National University Medical Research Center, Seoul, Korea
- 3Integrated Major in Innovative Medical Science, Seoul National University Graduate School, Seoul, Korea
- 4Department of Clinical Pharmacology and Therapeutics, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2542805
- DOI: http://doi.org/10.3346/jkms.2023.38.e173
Abstract
- Background
Carvedilol is a beta-adrenergic receptor antagonist primarily metabolized by cytochromes P450 (CYP) 2D6. This study established a carvedilol population pharmacokinetic (PK)–pharmacodynamic (PD) model to describe the effects of CYP2D6 genetic polymorphisms on the inter-individual variability of PK and PD.
Methods
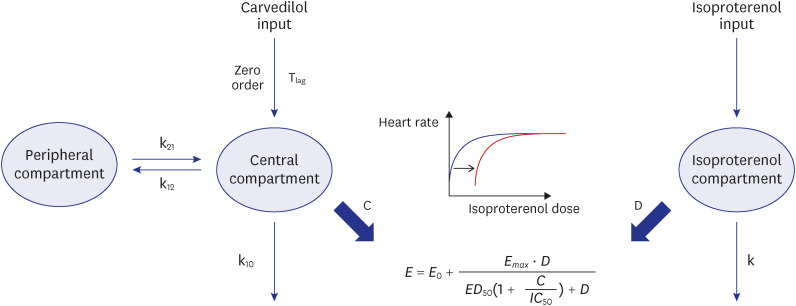
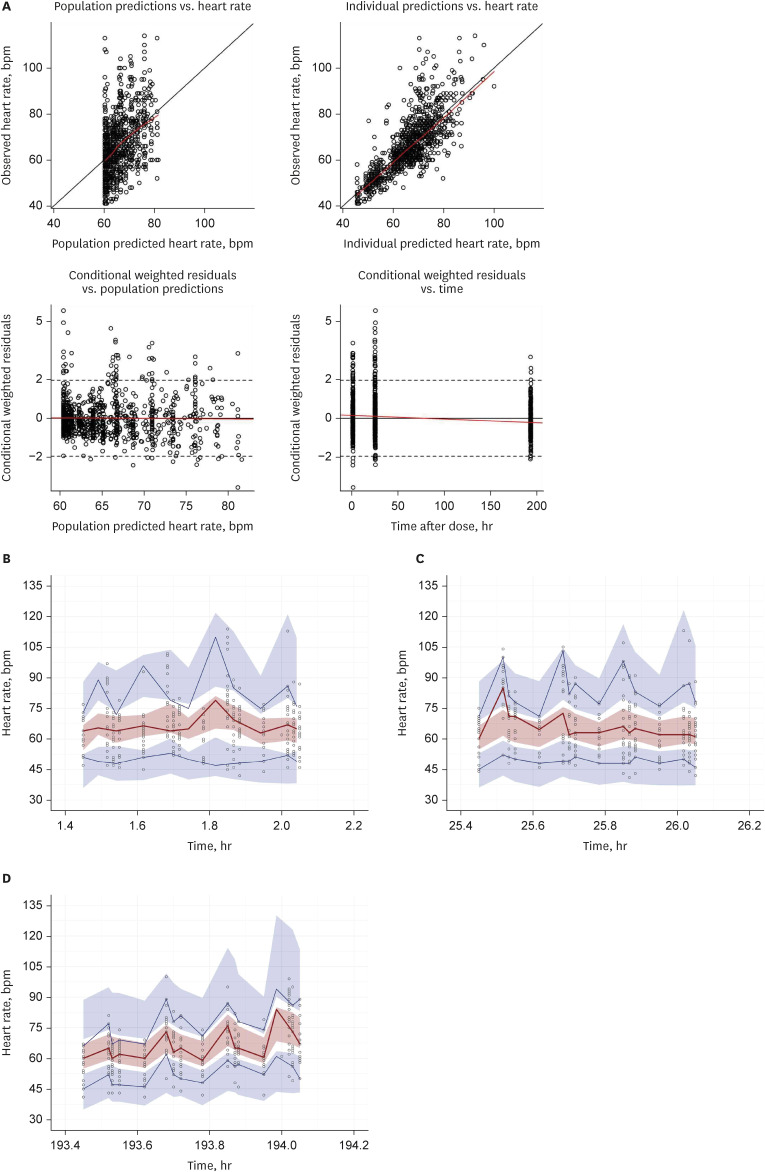
The PK–PD model was developed from a clinical study conducted on 21 healthy subjects divided into three CYP2D6 phenotype groups, with six subjects in the extensive metabolizer (EM, *1/*1, *1/*2), seven in the intermediate metabolizer-1 (IM-1, *1/*10, *2/*10), and eight in the intermediate metabolizer-2 (IM-2, *10/*10) groups. The PK–PD model was sequentially developed, and the isoproterenol-induced heart rate changes were used to establish the PD model. A direct effect response and inhibitory E max model were used to develop a carvedilol PK–PD model.
Results
The carvedilol PK was well described by a two-compartment model with zeroorder absorption, lag time, and first-order elimination. The carvedilol clearance in the CYP2D6*10/*10 group decreased by 32.8% compared with the other groups. The inhibitory concentration of carvedilol estimated from the final PK–PD model was 16.5 ng/mL regardless of the CYP2D6 phenotype.
Conclusion
The PK–PD model revealed that the CYP2D6 genetic polymorphisms were contributed to the inter-individual variability of carvedilol PK, but not PD.
Keyword
Figure
Reference
-
1. Ruffolo RR Jr, Gellai M, Hieble JP, Willette RN, Nichols AJ. The pharmacology of carvedilol. Eur J Clin Pharmacol. 1990; 38(Suppl 2):S82–S88. PMID: 1974511.
Article2. Oldham HG, Clarke SE. In vitro identification of the human cytochrome P450 enzymes involved in the metabolism of R(+)- and S(-)-carvedilol. Drug Metab Dispos. 1997; 25(8):970–977. PMID: 9280405.3. U.S. Food and Drug Administration. Coreg (carvedilol) [package insert]. Updated 2017. Accessed May 7, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020297s038lbl.pdf .4. Stafylas PC, Sarafidis PA. Carvedilol in hypertension treatment. Vasc Health Risk Manag. 2008; 4(1):23–30. PMID: 18629377.
Article5. Morgan T. Clinical pharmacokinetics and pharmacodynamics of carvedilol. Clin Pharmacokinet. 1994; 26(5):335–346. PMID: 7914479.
Article6. Tenero D, Boike S, Boyle D, Ilson B, Fesniak HF, Brozena S, et al. Steady-state pharmacokinetics of carvedilol and its enantiomers in patients with congestive heart failure. J Clin Pharmacol. 2000; 40(8):844–853. PMID: 10934668.
Article7. Gehr TW, Tenero DM, Boyle DA, Qian Y, Sica DA, Shusterman NH. The pharmacokinetics of carvedilol and its metabolites after single and multiple dose oral administration in patients with hypertension and renal insufficiency. Eur J Clin Pharmacol. 1999; 55(4):269–277. PMID: 10424319.
Article8. Taylor C, Crosby I, Yip V, Maguire P, Pirmohamed M, Turner RM. A review of the important role of CYP2D6 in pharmacogenomics. Genes (Basel). 2020; 11(11):1295. PMID: 33143137.
Article9. Byeon JY, Kim YH, Lee CM, Kim SH, Chae WK, Jung EH, et al. CYP2D6 allele frequencies in Korean population, comparison with East Asian, Caucasian and African populations, and the comparison of metabolic activity of CYP2D6 genotypes. Arch Pharm Res. 2018; 41(9):921–930. PMID: 30191460.
Article10. Lee SY, Sohn KM, Ryu JY, Yoon YR, Shin JG, Kim JW. Sequence-based CYP2D6 genotyping in the Korean population. Ther Drug Monit. 2006; 28(3):382–387. PMID: 16778723.
Article11. Neafsey P, Ginsberg G, Hattis D, Sonawane B. Genetic polymorphism in cytochrome P450 2D6 (CYP2D6): population distribution of CYP2D6 activity. J Toxicol Environ Health B Crit Rev. 2009; 12(5-6):334–361. PMID: 20183526.
Article12. Jung E, Ryu S, Park Z, Lee JG, Yi JY, Seo DW, et al. Influence of CYP2D6 polymorphism on the pharmacokinetic/pharmacodynamic characteristics of carvedilol in healthy Korean volunteers. J Korean Med Sci. 2018; 33(27):e182. PMID: 29962926.
Article13. Derendorf H, Lesko LJ, Chaikin P, Colburn WA, Lee P, Miller R, et al. Pharmacokinetic/pharmacodynamic modeling in drug research and development. J Clin Pharmacol. 2000; 40(12 Pt 2):1399–1418. PMID: 11185661.
Article14. Höcht C, Bertera FM, Mauro JS, Parola L, Taira CA. PK/PD modeling of β-Blockers in cardiovascular disease: an update. Int J Pharmacokinet. 2016; 1(1):55–68.
Article15. Tenero DM, Henderson LS, Campanile AM, Baidoo CA, Boyle D. Development of a pharmacokinetic/pharmacodynamic model for carvedilol to predict beta1-blockade in patients with congestive heart failure. Am J Cardiol. 2006; 98(7A):27L–31L.
Article16. Baek IH, Yun MH, Yun HY, Kwon KI. Pharmacokinetic/pharmacodynamic modeling of the cardiovascular effects of beta blockers in humans. Arch Pharm Res. 2008; 31(6):814–821. PMID: 18563366.
Article17. Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003; 30(6):387–404. PMID: 15000421.
Article18. Upton RN, Mould DR. Basic concepts in population modeling, simulation, and model-based drug development: part 3-introduction to pharmacodynamic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2014; 3(1):e88. PMID: 24384783.
Article19. Arnold JM, McDevitt DG. Heart rate and blood pressure responses to intravenous boluses of isoprenaline in the presence of propranolol, practolol and atropine. Br J Clin Pharmacol. 1983; 16(2):175–184. PMID: 6137231.
Article20. Gabrielsson J, Weiner D. Pharmacokinetic and Pharmacokinetic Data Analysis: Concepts and Applications. 3rd ed. Stockholm, Sweden: Swedish Pharmaceutical Press;2000.21. Sehrt D, Meineke I, Tzvetkov M, Gültepe S, Brockmöller J. Carvedilol pharmacokinetics and pharmacodynamics in relation to CYP2D6 and ADRB pharmacogenetics. Pharmacogenomics. 2011; 12(6):783–795. PMID: 21599570.
Article22. Shihmanter R, Nulman I, Goland S, Caspi A, Bar-Haim A, Harary I, et al. Variation in the CYP2D6 genotype is not associated with carvedilol dose changes in patients with heart failure. J Clin Pharm Ther. 2014; 39(4):432–438. PMID: 24673480.
Article23. Nikolic VN, Jankovic SM, Velickovic-Radovanović R, Apostolović S, Stanojevic D, Zivanovic S, et al. Population pharmacokinetics of carvedilol in patients with congestive heart failure. J Pharm Sci. 2013; 102(8):2851–2858. PMID: 23728853.
Article24. Saito M, Kawana J, Ohno T, Hanada K, Kaneko M, Mihara K, et al. Population pharmacokinetics of R- and S-carvedilol in Japanese patients with chronic heart failure. Biol Pharm Bull. 2010; 33(8):1378–1384. PMID: 20686235.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of CYP2D6 Polymorphism on the Pharmacokinetic/Pharmacodynamic Characteristics of Carvedilol in Healthy Korean Volunteers
- Drug Interaction in New Antipsychotics
- Pharmacokinetic and pharmacodynamic modeling in anesthetic field
- A semi-compartmental model describing the pharmacokinetic-pharmacodynamic relationship
- Obesity and anesthetic pharmacology: simulation of target-controlled infusion models of propofol and remifentanil