J Stroke.
2023 May;25(2):242-250. 10.5853/jos.2022.02957.
Moderate-Intensity Rosuvastatin Plus Ezetimibe Versus High-Intensity Rosuvastatin for Target Low-Density Lipoprotein Cholesterol Goal Achievement in Patients With Recent Ischemic Stroke: A Randomized Controlled Trial
- Affiliations
-
- 1Department of Neurology, Ilsan Paik Hospital, Inje University, Goyang, Korea
- 2Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Neurology, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
- 4Department of Neurology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 5Department of Neurology, Seoul Hospital, Ewha Women’s University College of Medicine, Seoul, Korea
- 6Department of Neurology, Yonsei University College of Medicine, Seoul, Korea
- 7Department of Neurology, Inha University Hospital, Incheon, Korea
- 8Department of Neurology, Seoul National University College of Medicine, Seoul, Korea
- 9Department of Neurology, Kyung Hee University Medical Center, Seoul, Korea
- 10Department of Neurology, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 11Department of Neurology, Hallym University Sacred Heart Hospital, Anyang, Korea
- 12Department of Neurology, Chung-Ang University Hospital, Seoul, Korea
- 13Department of Neurology, Korea University Guro Hospital, Seoul, Korea
- 14Department of Biostatistics, Korea University College of Medicine, Seoul, Korea
- KMID: 2542475
- DOI: http://doi.org/10.5853/jos.2022.02957
Abstract
- Background and Purpose
Moderate-intensity statin plus ezetimibe versus high-intensity statin alone may provide a greater low-density lipoprotein cholesterol (LDL-C) reduction in patients with recent ischemic stroke.
Methods
This randomized, open-label, controlled trial assigned patients with recent ischemic stroke <90 days to rosuvastatin/ezetimibe 10/10 mg once daily (ROS10/EZT10) or to rosuvastatin 20 mg once daily (ROS20). The primary endpoint was LDL-C reduction ≥50% from baseline at 90 days. Key secondary endpoints were LDL-C <70 mg/dL and multiple lipid goal achievement, and composite of major vascular events.
Results
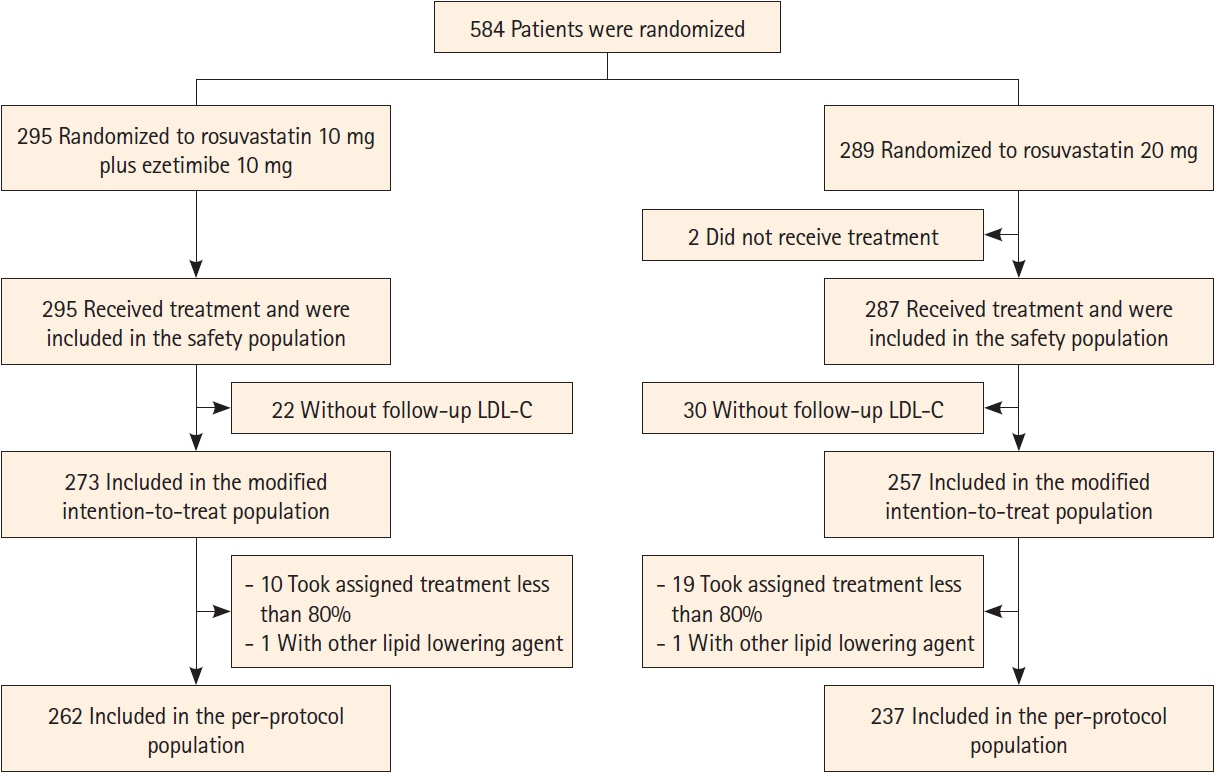
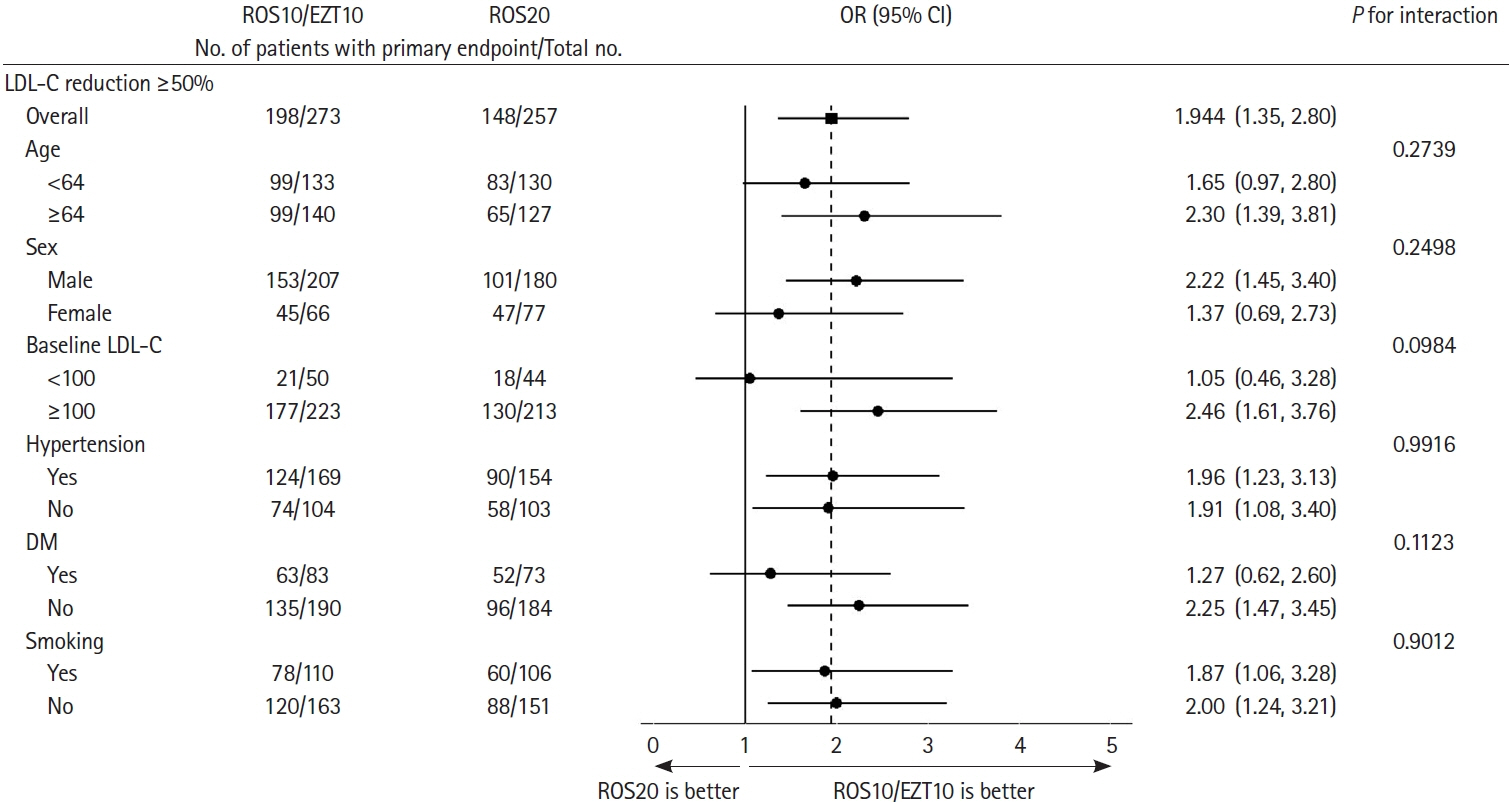
Of 584 randomized, 530 were included in the modified intention-to-treat analysis. The baseline LDL-C level was 130.2±34.7 mg/dL in the ROS10/EZT10 group and 131.0±33.9 mg/dL in the ROS20 group. The primary endpoint was achieved in 198 patients (72.5%) in the ROS10/EZT10 group and 148 (57.6%) in the ROS20 group (odds ratio [95% confidence interval], 1.944 [1.352–2.795]; P= 0.0003). LDL-C level <70 mg/dL was achieved in 80.2% and 65.4% in the ROS10/EZT10 and ROS20 groups (P=0.0001). Multiple lipid goal achievement rate was 71.1% and 53.7% in the ROS10/EZT10 and ROS20 groups (P<0.0001). Major vascular events occurred in 1 patient in the ROS10/EZT10 group and 9 in the ROS20 group (P=0.0091). The adverse event rates did not differ between the two groups.
Conclusion
Moderate-intensity rosuvastatin plus ezetimibe was superior to high-intensity rosuvastatin alone for intensive LDL-C reduction in patients with recent ischemic stroke. With the combination therapy, more than 70% of patients achieved LDL-C reduction ≥50% and 80% had an LDL-C <70 mg/dL at 90 days.
Keyword
Figure
Reference
-
References
1. Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009; 8:453–463.
Article2. Shin J, Chung JW, Jang HS, Lee J, Hong KS, Bang OY, et al. Achieved low-density lipoprotein cholesterol level and stroke risk: a meta-analysis of 23 randomised trials. Eur J Prev Cardiol. 2021; 28:905–916.
Article3. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016; 37:2999–3058.
Article4. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021; 52:e364–e467.
Article5. Alsheikh-Ali AA, Maddukuri PV, Han H, Karas RH. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007; 50:409–418.
Article6. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670–1681.
Article7. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011; 305:2556–2564.
Article8. Nicholls SJ, Brandrup-Wognsen G, Palmer M, Barter PJ. Metaanalysis of comparative efficacy of increasing dose of atorvastatin versus Rosuvastatin versus Simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am J Cardiol. 2010; 105:69–76.
Article9. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017; 376:1713–1722.
Article10. Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2017; 2:547–555.
Article11. Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017; 390:1962–1971.
Article12. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387–2397.
Article13. Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997; 283:157–163.14. Morrone D, Weintraub WS, Toth PP, Hanson ME, Lowe RS, Lin J, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012; 223:251–261.
Article15. Ambegaonkar BM, Tipping D, Polis AB, Tomassini JE, Tershakovec AM. Achieving goal lipid levels with ezetimibe plus statin add-on or switch therapy compared with doubling the statin dose. A pooled analysis. Atherosclerosis. 2014; 237:829–837.
Article16. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014; 129(25 Suppl 2):S1–S45.17. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–2236.
Article18. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017; 23(Suppl 2):1–87.
Article19. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989; 46:1121–1123.20. Kim KJ, Kim SH, Yoon YW, Rha SW, Hong SJ, Kwak CH, et al. Effect of fixed-dose combinations of ezetimibe plus rosuvastatin in patients with primary hypercholesterolemia: MRS-ROZE (Multicenter Randomized Study of ROsuvastatin and eZEtimibe). Cardiovasc Ther. 2016; 34:371–382.
Article21. Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022; 400:380–390.
Article22. Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020; 382:9–19.
Article23. Amarenco P, Kim JS, Labreuche J, Charles H, Giroud M, Lee BC, et al. Yield of dual therapy with statin and ezetimibe in the Treat Stroke to Target trial. Stroke. 2022; 53:3260–3267.
Article24. Heo JH, Song D, Nam HS, Kim EY, Kim YD, Lee KY, et al. Effect and safety of rosuvastatin in acute ischemic stroke. J Stroke. 2016; 18:87–95.
Article25. Tamargo J, Kaski JC, Kimura T, Barton JC, Yamamoto K, Komiyama M, et al. Racial and ethnic differences in pharmacotherapy to prevent coronary artery disease and thrombotic events. Eur Heart J Cardiovasc Pharmacother. 2022; 8:738–751.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipid-Lowering Efficacy of Combination Therapy With ModerateIntensity Statin and Ezetimibe Versus High-Intensity Statin Monotherapy: A Randomized, Open-Label, NonInferiority Trial From Korea
- The Efficacy and Safety of Moderate-Intensity Rosuvastatin with Ezetimibe versus High-Intensity Rosuvastatin in High Atherosclerotic Cardiovascular Disease Risk Patients with Type 2 Diabetes Mellitus: A Randomized, Multicenter, Open, Parallel, Phase 4 Study
- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review
- Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus
- Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)