Cancer Res Treat.
2023 Apr;55(2):704-705. 10.4143/crt.2022.1524.
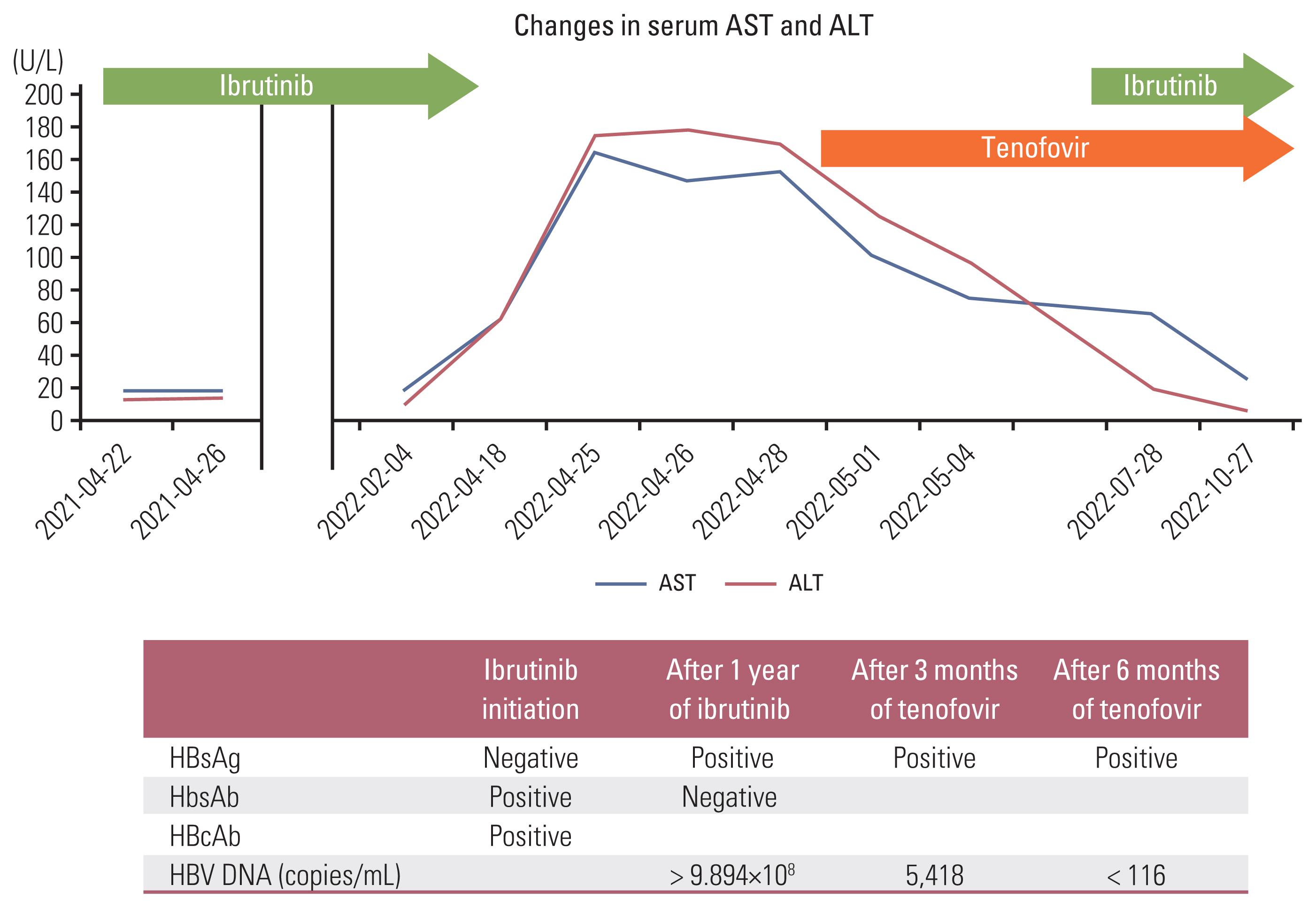
Hepatitis B Virus Reactivation in a Chronic Lymphocytic Leukemia Patient Treated with Ibrutinib
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- KMID: 2541257
- DOI: http://doi.org/10.4143/crt.2022.1524
Figure
Reference
-
References
1. Iskender G, Iskender D, Ertek M. Hepatitis B virus reactivation under ibrutinib treatment in a patient with chronic lymphocytic leukemia. Turk J Haematol. 2020; 37:208–9.2. Innocenti I, Reda G, Visentin A, Coscia M, Motta M, Murru R, et al. Risk of hepatitis B virus reactivation in chronic lymphocytic leukemia patients receiving ibrutinib with or without antiviral prophylaxis: a retrospective multicentric GIMEMA study. Haematologica. 2022; 107:1470–3.
Article3. Hammond SP, Chen K, Pandit A, Davids MS, Issa NC, Marty FM. Risk of hepatitis B virus reactivation in patients treated with ibrutinib. Blood. 2018; 131:1987–9.
Article4. Yang S, Zhu R, Li N, Feng Y, Zuo R, Gale RP, et al. Ibrutinib in advanced vhronic lymphocytic leukemia/small lymphocytic lymphoma: lower risk of hepatitis B virus reactivation. Acta Haematol. 2022; 145:54–62.
Article5. Molica S, Levato L, Mirabelli R, Tedeschi A, Lentini M. Feasibility and safety of therapy with ibrutinib after antiviral control of hepatitis B virus (HBV) reactivation in chronic lymphocytic leukemia patients. Leuk Lymphoma. 2018; 59:2734–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatitis B virus reactivation during chlorambucil and prednisolone treatment in an HBsAg-negative and anti-HBs-positive patient with B-cell chronic lymphocytic leukemia
- Hepatitis B Virus Reactivation after Partial Hepatic Irradiation Alone: A Case Report
- Progressive Multifocal Leukoencephalopathy after Ibrutinib Therapy for Chronic Lymphocytic Leukemia
- Prevention of Viral Hepatitis and Vaccination
- Safety and efficacy analysis of ibrutinib in 32 patients with CLL and various B-cell lymphomas: real-world data from a single-center study in Turkey